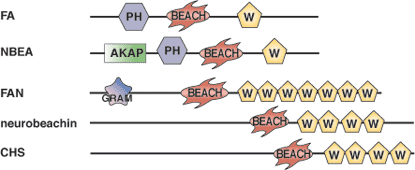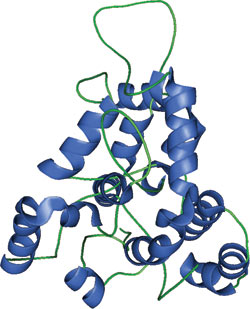
BEACH domain of human neurobeachin.
The BEACH domain was originally discovered as a conserved region within the protein responsible for the Chediak-Higashi syndrome (CHS). The name BEACH comes from "beige and CHS" domain, where “beige” is another name for CHS disease in mice. The BEACH domain consists of approximately 300 amino acids and is found in proteins involved in vesicle trafficking, membrane dynamics and receptor signaling. Although the specific function of the BEACH domain remains unclear, structural studies have uncovered a weakly conserved, yet structurally identifiable, PH domain approximately 100 residues prior to the BEACH domain. This PH domain interacts with the BEACH domain to form a single unit. A large groove found between the PH and BEACH domains may serve as a ligand-binding site. The BEACH domain is usually followed by a series of WD repeats.
The BEACH domain adopts a unique and unusual structure, where large segments of the domain are either buried or help enclose the hydrophobic core. These seven, partially extended segments (e1 to e7) are not β-strands, as they are not fully extended. Segments e1, e4 and e7 contain regions that are completely buried in the domain core and contain the most conserved residues within the BEACH domain. The BEACH domain also contains 11 α-helices (α A to α K) arranged on the periphery of the structure that enclose the core of the domain.