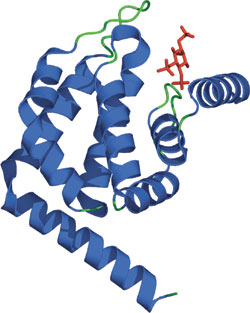
The ENTH domain of Epsin bound PtdIns(1,4,5)P3.
The Epsin NH2-Terminal Homology (ENTH) domain is a membrane binding motif of approximately 150 amino acids that was first identified in the endocytotic protein epsin 1. Proteins containing this domain can bind to phospholipids including PtdIns(4,5)P2 and PtdIns(1,4,5)P3. This suggests that ENTH domain containing proteins act as clathrin adaptors during endocytosis through ENTH domain binding to the phospholipid bilayer, allowing recruitment of clathrin components and clathrin accessory factors to the cell membrane. A pair of ENTH containing proteins (HIP1, HIP1R) localizes to clathrin-coated pits and contains a putative actin-binding motif (ILWEQ), which may couple the actin cytoskeleton and endocytosis.
Despite low primary sequence similarity, the solved structures of various ENTH domains are very similar and are composed of eight α helices connected by loops of varying lengths. Three helical hairpins (α1-2, α3-4, and α6-7) are stacked consecutively with a right-handed twist, conferring a rectangular topology to the ENTH domain. A cleft of positively charged residues contributes to phoshoinositide binding. Clusters of basic amino acids, present in some but not all ENTH family members (AP180/CALM), have been shown to enrich the affinity for phosphoinositides.
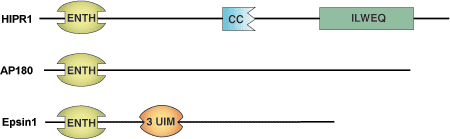
| ENTH Domain Proteins | Binding Partners |
| Epsin1 | PtdIns(4,5)P2 / PtdIns(1,4,5)P3 |
| AP180 | PtdIns(4,5)P2 / PtdIns(1,4,5)P3 |
| HIP1R | PI(3,4)P2; PI(3,5)P2 |