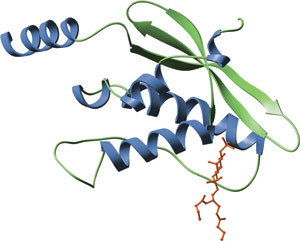
The PX domain of human p40phox bound to PI(3)P (red).
The Phox homology (PX) domain is a phospholipid-binding domain consisting of ~120 amino acids. The PX domain is found in more than 250 proteins, including the p40phox and p47phox components of the NADPH oxidase complex, sorting nexins, phospholipases D1 and 2 and the kinases PI3K and CISK. PX domains contain an N-terminal, three-stranded β-sheet followed by a helical subdomain made up of four α-helices. PX domains preferentially bind to PtdIns(3)P, although some have been reported to bind other phosphoinositides. Proteins containing PX domains have been implicated in a wide spectrum of functions, including protein sorting, vesicular trafficking and phospholipid metabolism.
The structural basis for PX domain interactions with phosphoinositides have been determined from the structures of the PX domains of p40phox and Vmp7p in a complex with PI(3)P. Interactions between all PX domains and their bound phosphoinositides involve the 1-phosphate and the inositol ring. A hydrogen bond between a well-conserved K92 residue (in p40phox) and the 1-phosphate is the most important interaction with this group. Y59 (in p40phox) forms the floor of the lipid-binding pocket in the PX domain, allowing the inositol ring of PI(3)P to stack tightly against it. This carbohydrate-aromatic stacking is likely to be a common feature of binding, since all PX domains have either Y or F at the analogous position.
| PX Domain Proteins | Binding Partners |
| p40phox | PI(3)P |
| p47phox | PI(3,4)P2, PI(3)P |
| Vmp7 | PI(3)P |
| SNX | PI(3,5)P2, PI(4)P, PI(5)P |
| PLD1 | PI(3,4,5)P3 |
| CISK | PI(3,4,5)P3 |
| PI3K (Class II) |