Our flow-validated products undergo rigorous testing in biologically relevant models, ensuring specificity and an optimal signal-to-noise ratio (S/N) for both conjugated and unconjugated antibodies. Cross-platform validation further confirms antibody specificity. In addition, all antibodies have been tested for optimal dilution, specificity, stability and lot-to-lot reproducibility to ensure they work the first time, every time.
Our thoroughly validated antibodies for flow cytometry make it possible to examine complex intracellular signaling cascades in cell lines, dissociated tissues, aspirates, or hematology specimens, and trust the accuracy of your results.
The performance of our antibodies is routinely validated across multiple platforms. Below is an example, which demonstrates why such testing is necessary.
Comparative analysis in flow cytometry and immunofluorescence confirms that the CST antibody detects the target specifically. The signal correctly localizes to the nuclear compartment of the cell, although the signal is less robust.
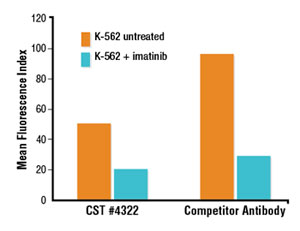
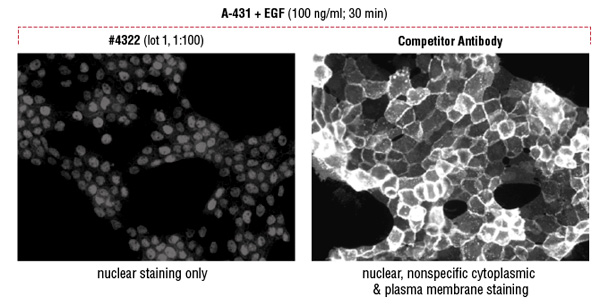
Flow cytometric analysis suggests a brighter signal from a competitor’s Phospho-Stat5 (Tyr694) antibody compared to a lower fold induction with Phospho-Stat5 (Tyr694) (D47E7) XP® Rabbit mAb #4322 (A). However, immunofluorescent analysis reveals that the competitor antibody inappropriately stains the cytoplasm and plasma membrane, while #4322 demonstrates only the appropriate nuclear staining (B).
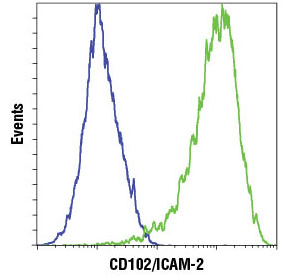
Flow cytometric analysis of HeLa cells (blue) and HUVEC (green) using CD102/ICAM-2 (D7P2Q) Rabbit mAb #13355. Anti-rabbit IgG (H+L), F(ab')2 Fragment (Alexa Fluor® 647 Conjugate) #4414 was used as a secondary antibody.
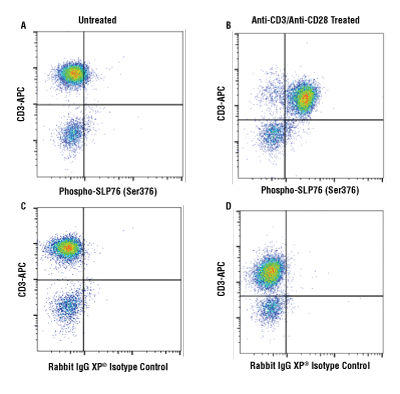
Flow cytometric analysis of human PBMCs untreated (A, C) or treated with cross-linked anti-CD3 plus anti-CD28 (10μg/ml each, 15 min; B, D), using Phospho-SLP-76 (Ser376) (D7S1K) XP® Rabbit mAb #92711 (A, B) or concentration-matched Rabbit (DA1E) mAb IgG XP® Isotype Control #3900 (C, D), and co-stained with a CD3 antibody. Anti-rabbit IgG (H+L), F(ab') 2 Fragment (Alexa Fluor® 488 Conjugate) #4412 was used as a secondary antibody.
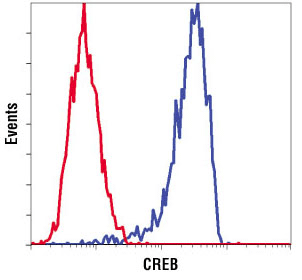
Flow cytometric analysis of SH-SY5Y cells using CREB (D76D11) Rabbit mAb Antibody #4820 (blue) compared to concentration matched #3900 Rabbit (DA1E) mAb IgG XP® Isotype Control #3900 (red).
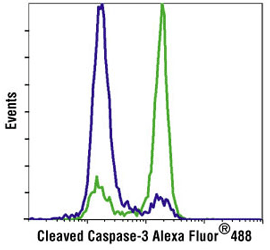
Flow cytometric analysis of Jurkat cells, untreated (blue) or treated with Etoposide #2200 (green), using Cleaved Caspase-3 (Asp175) Antibody (Alexa Fluor® 488 Conjugate) #9669.
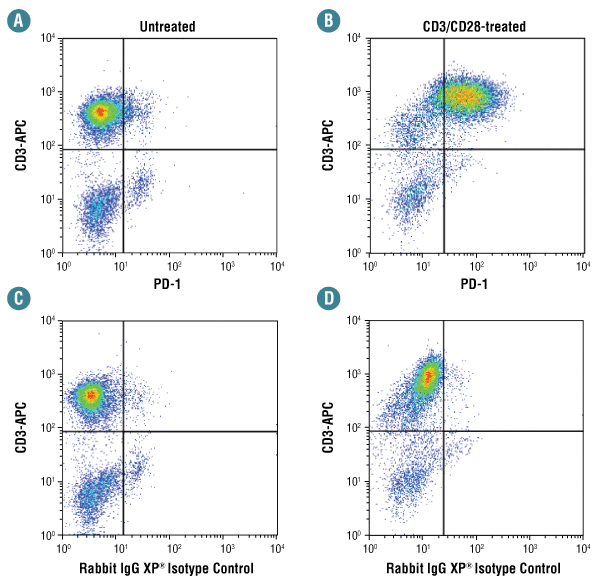
Flow cytometric analysis of human PBMCs untreated (A, C) or treated with anti-CD3/CD28 (72 hr; B, D), using PD-1 (D4W2J) XP® Rabbit mAb #86163 (A, B) compared to concentration-matched Rabbit (DA1E) mAb IgG XP® Isotype Control #3900 (C, D), and co-stained with a CD3 antibody.
CST determines the optimal concentration and staining protocols for each antibody.
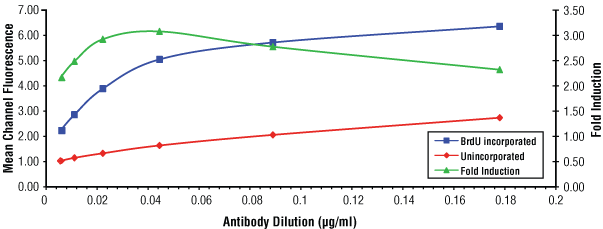
Flow cytometric analysis of Jurkat cells, unincorporated (red) or after 30 minutes of BrdU incorporation (blue), using serial dilutions of BrdU (Bu20a) Mouse mAb #5292. The fold-induction ratio is shown in green. Optimal concentration of #5292 was determined to be 0.044 µg/ml.
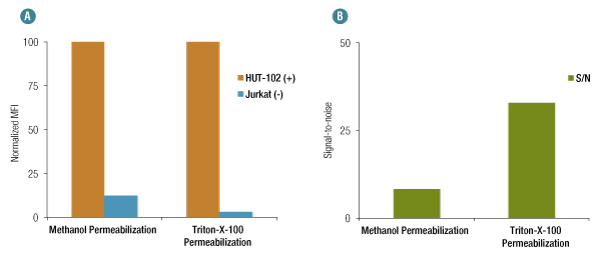
When using methanol permeabilization, OX40 (D1S6L) Rabbit mAb #15123, exhibits nonspecific binding in Jurkat cells (negative control) as compared to HUT-102 (positive control) (A). However, nonspecific binding is significantly reduced when Triton X-100 is used for permeabilization, generating a much higher S/N for the same concentration of the antibody (B).
Btk (D3H5) Rabbit mAb selectively isolates B cells from human whole blood. 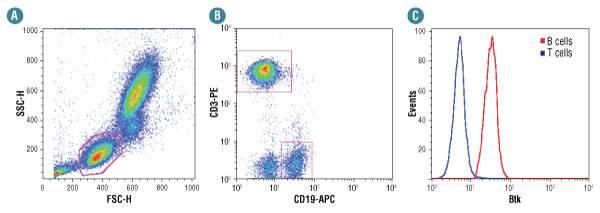
Human whole blood was fixed, lysed, and permeabilized per the CST Flow Alternate Protocol and stained using Btk (D3H5) Rabbit mAb #8547. Cells were gated as shown in (A). Samples were co-stained using CD3-PE and CD19-APC to distinguish T and B cell subpopulations, respectively (B). B (red) and T (blue) cell population gates were applied to a histogram depicting the mean fluorescence intensity of Btk (C). Anti-rabbit IgG (H+L), F(ab')2 Fragment (Alexa Fluor® 488 Conjugate) #4412 was used as a secondary antibody.