The goal of an immunohistochemistry (IHC) experiment is to visualize your target of interest in tissue, while maintaining the spatial context of the tissue architecture. Analysis of IHC results can be performed, most commonly, by eye or by utilizing software.
Depending on the antibody detection, IHC samples can be visualized using light or fluorescence microscopy.
In the case of fluorescence microscopy, and if additional detail is warranted, confocal or multispectral imaging systems can be used. For small antibody panel sizes (1-3 targets), conventional cameras are capable of providing robust images. Large panel sizes (more than 4 targets), require multispectral cameras. Multispectral imaging systems support software enabling linear unmixing of fluorophores (and chromogens) that have overlapping excitation/emission spectra and, thus, promote multiplex target detection with superior resolution.
See below for a comparison of wide-field images taken with a multispectral vs a conventional camera.
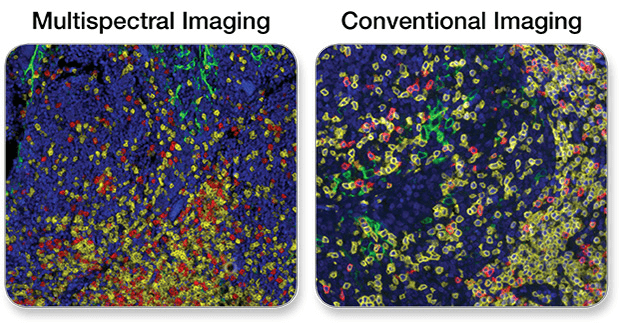
PD-L1, CD3ε, CD8α Multiplex IHC Panel #65713: A 3-plex Fluorescent mIHC analysis of paraffin-embedded human breast cancer using PD-L1 (E1L3N®) XP® Rabbit mAb #13684 (green), CD3ε (D7A6E™) XP® Rabbit mAb #85061 (yellow), and CD8α (C8/144B) Mouse mAb (IHC Specific) #70306 (Red). Blue pseudocolor = DAPI #8961 (fluorescent DNA dye).
The determination of target specificity in immunohistochemical analysis requires multiple validation steps. CST scientists use a variety of methods, as appropriate, to validate each IHC-recommended antibody, ensuring that the staining you observe is specific.
Several of the variables that should be considered are signal strength, background, and proper localization. Taken together, these work as indicators to inform the assessment of positive or negative results.
For more details on how CST ensures staining with our IHC antibodies is correct and specific, click here.
There are a number of variables, ranging from sample storage to reagent quality, which can impact the signal strength and background of your IHC experiment.
It goes without saying that you need a strong and specific primary antibody validated for IHC. However, the reagents you choose beyond the primary antibody can also have a significant impact on the strength of signal in your IHC experiment. Ensuring you work with recommended dilutions and reagents from the antibody-specific protocol for IHC, such SignalStain® Antibody Diluent #8112, SignalStain® Boost Detection Reagents, HRP for rabbit #8114 or mouse #8125 and SignalStain® DAB Substrate Kit #8059 can improve the signal strength of your assay.
In addition to producing a strong signal, it is important to decrease background staining. Using a polymer-based detection reagent, such as SignalStain® Boost Detection Reagents, HRP for rabbit #8114 or mouse #8125 can significantly cut down on background staining from endogenous biotin, thus improving your results. In addition, proper blocking with 1X TBST #9997 with 5% Normal Goat Serum #5425 for 30 minutes prior to incubation with the primary antibody can be crucial.
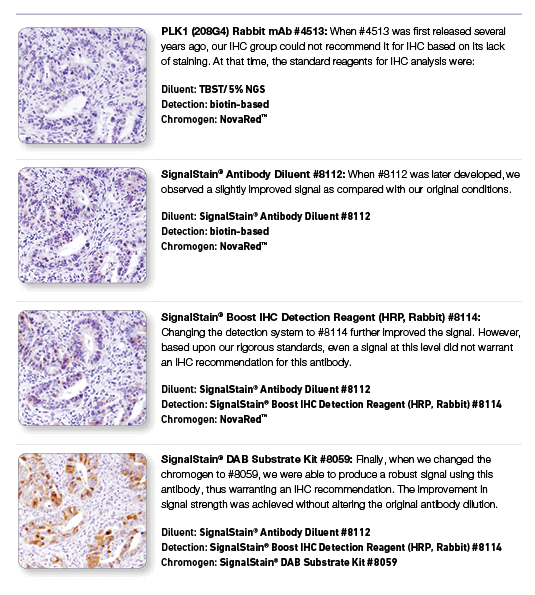
PLK1 (208G4) Rabbit mAb #4513: IHC analysis of paraffin-embedded human colon carcinoma using #4513 with step-by-step reagent substitution, as indicated, demonstrating that reagents can make the difference between poor staining and publishable results.
One of the primary benefits of IHC analysis is the ability to see your target while maintaining the spatial context of the tissue, including localization in appropriate cell types or subcellular compartments.
Ensuring proper localization of staining is an important aspect of IHC antibody validation at CST, as it should be of any IHC experiment. Resources such as The Human Protein Atlas and orthogonal approaches such as RNA data can be helpful in cases where proper localization is unknown.
Making a confident assessment of positive or negative results requires synthesis of information from your staining - such as signal strength, background, and localization. Another valuable resource to assess the performance of staining reagents and methods is the use of IHC control slides.
Control slides contain formalin-fixed, paraffin-embedded cell pellets as controls for immunohistochemistry protocols. Each slide contains a negative and a positive pellet, and each set contains 5 slides.
To browse IHC control slides available from CST, click here.