
The rat synaptic fusion complex comprising Syntaxin-1A (red), Synaptobrevin-II (blue) and SNAP-25B (green).
The mechanism by which a vesicle fuses with a specific membrane target appears to involve a highly conserved set of proteins called SNAREs (Soluble NSF Attachment protein [SNAP] Receptors). SNARE proteins are thought to mediate most or possibly all cellular membrane fusion events. Most SNAREs are C-terminally anchored integral membrane proteins capable of entering into a coiled-coil interaction with other SNARE proteins. The SNARE domain is a homologous domain of approximately 60 amino acids common to all SNARE proteins. The SNARE domain acts as a protein-protein interaction module in the assembly of a SNARE protein complex. The largely unstructured, monomeric SNARE motifs assemble into a protease resistant core complex. Different SNARE family members are distributed on distinct membranes throughout the cell, suggesting they may play a role in targeting during vesicular transport. However, the formation of SNARE core complexes appears to be rather promiscuous with little specificity.
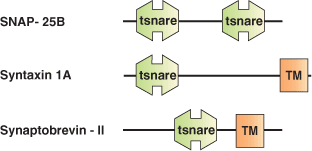
| SNARE complexes | SNARE domain proteins in complexes |
| Rat Synaptic Fusion SNARE Complex | Syntaxin-1A (Sx), Synaptobrevin-II (Sb), SNAP-25B |
| Yeast Exocytic post-Golgi SNARE Complex | Snc2, Sso1, Sec9 |
| Rat Endosomal SNARE Complex | Syntaxin-7, Vti 1b, Syntaxin 8 |