To perform intracellular flow cytometry, fixation and permeabilization of cells is required to allow for antibody penetration and subsequent binding to targets without disrupting cellular morphology.
Below we discuss several key factors, which will ensure successful experimental design particularly when studying post-translational modifications (PTMs).
Flow cytometric analysis of PTMs, such as phosphorylation, requires fixation of cells within a critical window of pathway activation. Fixation using 4% formaldehyde (methanol-free) optimally crosslinks intracellular proteins and any associated modifications. Furthermore, enzymatic activity responsible for removing modifications such as phosphatases can be de-activated. A lower concentration of formaldehyde may be necessary for some targets if the extent of crosslinking masks the target epitope.
Permeabilization with ice-cold 90% methanol added dropwise maintains structural cell integrity while allowing efficient antibody penetration without denaturing PTM epitopes that have previously been aldehyde fixed (1).
Ex vivo stimulation of whole blood allows for perturbation of peripheral blood cells within the blood matrix, resulting in a more physiologically relevant model of pathway signaling and other cellular processes. Fixation in 4% formaldehyde stabilizes protein interactions and subsequent treatment with 0.1% Triton X-100 lyses red blood cells (RBCs) (1). This allows permeabilization with 50% methanol to preserve surface marker integrity, enabling the simultaneous analysis of both surface and intracellular targets
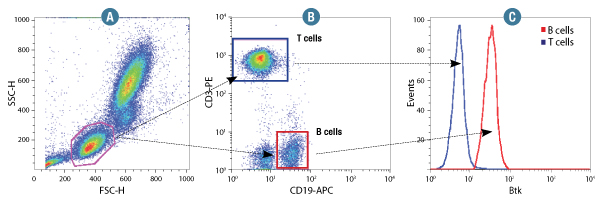
Btk (D3H5) Rabbit mAb #8547: Human whole blood was fixed and permeabilized as outlined in the CST Flow Alternative Protocol and stained using #8547. Forward scatter (FSC) and Side scatter (SSC) were used to gate on lymphocyte cells (A). Samples were co-stained using CD3-PE and CD19-APC to distinguish T and B cell subpopulations (B). B (red) and T (Blue) cell population gates were applied to a histogram depicting the mean fluorescence intensity of Btk (C).
It is always important to account for the complexity of cellular systems when designing an experiment. In some instances, basal modification levels may be high and therefore, stimulation of the pathway of interest does not provide any additional detectable signal, which can be interpreted as a false negative result. This is a common phenomenon with phosphorylated proteins. To address this issue, the fixed and permeabilized sample can be treated with phosphatase to remove phosphate groups from proteins and then examined for decreased signal that indicates high basal phosphorylation levels. Endogenous phosphorylation levels in live cells can be brought down by serum starvation or by kinase inhibitor treatment. Conversely, transient phosphorylation events may be difficult to capture due to endogenous phosphatase activity, in which case treatment with a phosphatase inhibitor can help preserve phosphorylation signal.
A | B | C |
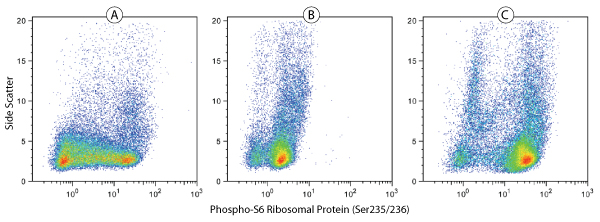
Phospho-S6 Ribosomal Protein (Ser235/236)(D57.2.2E) XP® Rabbit mAb (Alexa Fluor®488 Conjugate) #4803: Human whole blood was fixed and permeabilized as outlined in the CST Flow Alternate Protocol, and untreated (A), treated with λ phosphatase (B), or treated with TPA #4174 (C), before being stained using #4803.
Controls allow the investigator to determine whether or not the assay in question is working properly. Careful control design is especially critical for interpreting results from a flow cytometry experiment, given that a number of factors need to be considered. With this in mind, we recommend the following types of controls to be incorporated into your workflow:
The table below lists the appropriate controls relevant to each of the three categories above.
| Control | Why is it needed? |
|---|---|
| Unstained cells |
|
| Fully stained cells |
|
| Cell samples/beads stained with single fluorescent markers |
|
| Isotype control |
|
| Fluorescence Minus One (FMO) staining |
|
| Unstimulated or untreated sample |
|
| Positive cells (stimulated) |
|
| Cells stained with a viability dye, e.g., propidium iodide |
|