Intracellular flow cytometry can be used to measure unique signaling events to do with apoptosis, pluripotency status and epigenetic activity. These profiles can be analyzed in distinct cell types within a population, therefore allowing comparison of the working mechanisms within different cell types.
Flow cytometry helps correlate phenotype, based on cell surface marker expression, with functional output, e.g., cytokine release, production of transcription factors, or phosphorylation-based activation. Below are examples of intracellular marks selectively expressed in a specialized population of immune cells. In some instances these marks dictate the function and activation state of the cell.
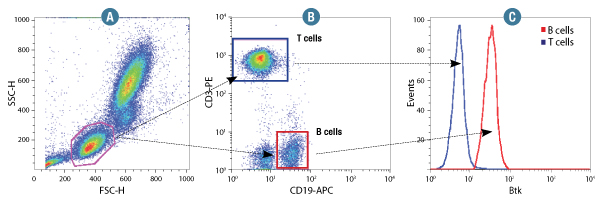
Btk (D3H5) Rabbit mAb #8547: Human whole blood was fixed and permeabilized as outlined in the CST Flow Alternative Protocol and stained using #8547. Forward scatter (FSC) and Side scatter (SSC) were used to gate on lymphocyte cells (A). Samples were co-stained using CD3-PE and CD19-APC to distinguish T and B cell subpopulations (B). B (red) and T (Blue) cell population gates were applied to a histogram depicting the mean fluorescence intensity of Btk (C).
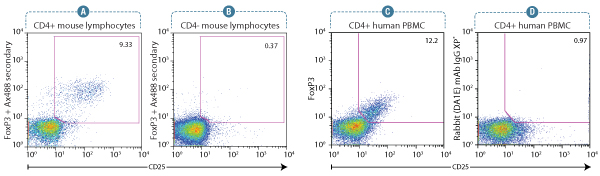
FoxP3 (D608R) XP® Rabbit mAb #12653: Flow cytometry analysis of murine spleen lymphocytes using a CD25 antibody and #12653. Anti-rabbit IgG (H+L), F(ab’)2 Fragment (Alexa Fluor® 488 Conjugate) #4412 was used as a secondary antibody. Analysis was performed on gated CD4+ lymphocytes (A) and CD4- lymphocytes (B) as an internal negative control. #12653 enables optimal detection of signal intensity and population frequency of FoxP3+/CD25+ Treg cells.
FoxP3 (D608C) XP® Rabbit mAb #12632: Flow cytometry analysis of human peripheral blood mononuclear cells gated on CD4+ lymphocytes, showing FoxP3 expression in CD25+ cells using #12632 (C), and corresponding absence of signal in CD25+ cells using concentration-matched Rabbit (DA1E) mAb IgG XP® Isotype Control #3900(D). #4412 was used as a secondary antibody. FoxP3 (D608C) Rabbit mAb #12632 clearly defines the FoxP3+/CD25+ subset with regards to signal intensity and population frequency.
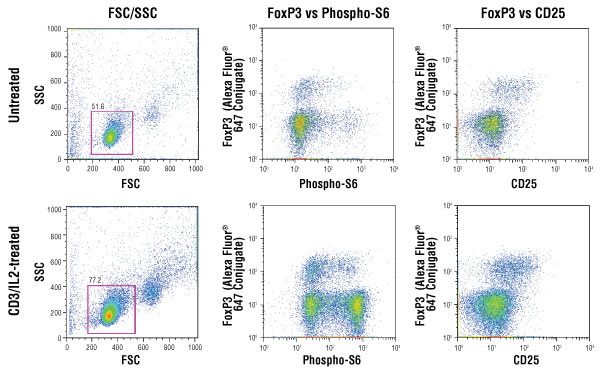
Isolated mouse splenocytes were stained for CD25 detection in the green channel, enriched for CD4+ T cells (gated cells) and either left untreated (top panels) or treated with IL-2 (20 ng/ml) on a CD3-coated plate (bottom panels). The cells were subsequently fixed, permeabilized and stained with FoxP3 (D608R) XP® rabbit mAb conjugated to Alexa Fluor® 647 #13399 and Phospho-S6 ribosomal protein (Ser235/236) (D57.2.2E) XP® rabbit mAb conjugated to PE #5316. Mouse splenocyte cell model system was kindly provided by Dr. Ryusuke Nakagawa, Department of Microbiology and Immunology, School of Medicine, Keio University, Shinjuku-ku, Tokyo, Japan.
Epigenetics is the study of histone and DNA modifications, which drive chromatin compaction and relaxation thus governing gene silencing and transcription, respectively.
An increasing number of research studies are utilizing flow cytometry as their readout for global epigenetic modifications (1-3). 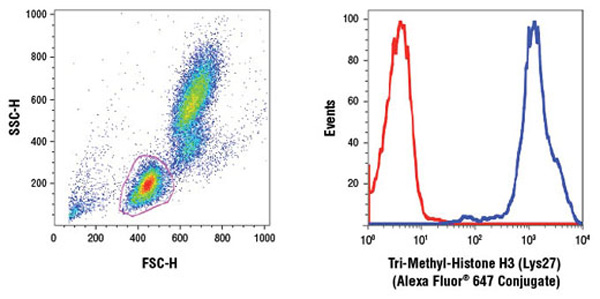
Human whole blood was fixed, and permeabilized as per Cell Signaling Technology Flow Alternate Protocol and stained using Tri-Methyl-Histone H3 (Lys27)(C36B11) Rabbit mAb (Alexa Fluor® 647 Conjugate) #12158. The forward/side-scatter lymphocyte gate was applied to a histogram depicting the mean fluorescence intensity of Tri-Methyl-Histone H3 (Lys27) (blue) and concentration-matched Rabbit (DA1E) mAb IgG XP® Isotype Control (Alexa Fluor® 647 Conjugate) #2985 (red).
Stem cells exhibit two unique properties: (1) potency, defined as the potential to differentiate along different cell lineages; and (2) the capacity for self-renewal, which allows them to be propagated indefinitely in culture.
Embryonic stem cells (ESCs) have the capacity to differentiate into any cell type of the body and are thus pluripotent in nature. Intracellular flow cytometry can be utilized to track the pluripotency status of ESCs in response to various biological cues (see example below).
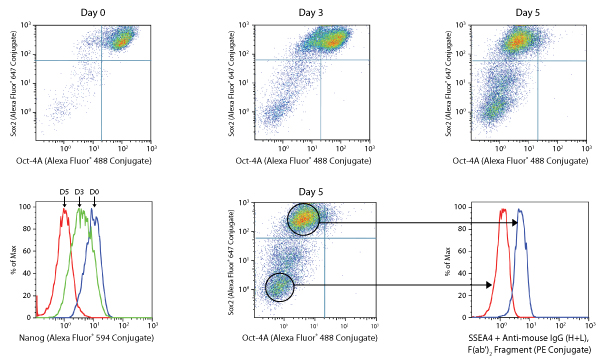
NTERA-2 differentiation assay.
NTERA-2 is a pluripotent human embryonal carcinoma cell line, which differentiates along neuroectodermal lineages in response to retinoic acid (RA). Exposure of these cells to RA (5 μM) over a 5-day period causes a gradual decline in the expression of Sox2 and Oct4 (dot plots, A), and Nanog and SSEA4 (histograms, B) pluripotency markers resulting in the appearance of a double negative fully differentiated neuronal cells.
Apoptosis is a regulated cellular suicide mechanism characterized by nuclear condensation, cell shrinkage, membrane blebbing, and DNA fragmentation. Caspases, a family of cysteine proteases, are the master regulators of this process.
Detection of apoptotic marks by flow cytometry, often in conjunction with the use of viability dyes, have become common practice in this field of research.

Annexin V-FITC Early Apoptosis Detection Kit #6592: Flow cytometric analysis of Jurkat cells untreated (left) or treated with camptothecin (10uM, 4 hr; right) using #6592.
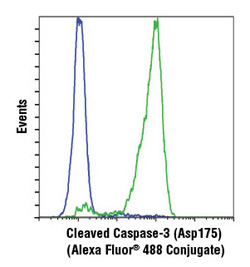
Cleaved Caspase-3 (Asp175) (D3E9) Rabbit mAb (Alexa Fluor® 488 Conjugate) #9603. Flow cytometric analysis of Jurkat cells, untreated (blue) or treated with etoposide #2200 (green), using #9603.