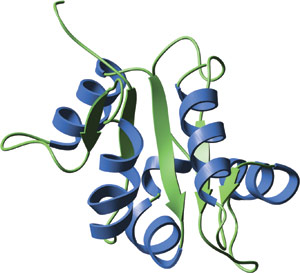
The TIR domain of human TLR2.
The Toll/Il-1 Receptor (TIR) domain was first characterized due to homology between the intracellular regions of the mammalian IL-1 receptor (IL-1R) and the Drosophila protein Toll. Subsequently, six Toll-Like Receptors (TLRs) have been identified in Drosophila and more than twenty TLRs and IL-1Rs have been recognized in humans. Several adaptor proteins containing TIR domains have also been described. The domain consists of three 'boxes' of conserved residues set in a core sequence ranging from 135 to 160 amino acids. Intervening residues may vary, as sequence conservation between domains is only 20–30%. Two interfaces are responsible for mediating TIR domain interactions, which include receptor/adaptor oligomerization and association between receptors and adaptors. TLR and IL-1R signaling pathways are key mediators of the innate immune response to bacteria and fungi in both Drosophila and mammals. TIR domain interactions between receptors and adaptors play a key role in activating conserved cellular signal transduction pathways in response to bacterial LPS, microbial and viral pathogens, cytokines and growth factors. Homotypic and heterotypic interactions are thought to mediate receptor signaling. Activation involves liberation of NF-κB resulting in lymphocyte activation, immunoglobulin isotype switching and expression of cytokines and their receptors.
The crystal structure of TIR domains from human TLR1 and TLR2 reveal a central five-stranded parallel β-sheet surrounded by a total of five helices on both sides. Conserved residues are located in the hydrophobic core and large insertions or deletions can be present in several loop regions of different TIR domains. The BB loop, containing three highly conserved residues, protrudes from a large conserved surface patch and is thought to mediate heterodimeric interactions with TIR domain-containing adaptor proteins.
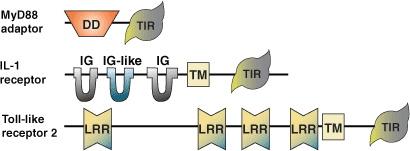
| TIR Domain Proteins | Binding Partners |
| TLR4 | Adaptors (MyD88, TIRAP, Mal), homotypic and possibly heterotypic (Toll-like receptors) interactions |
| MyD88 | Toll-like receptors, IL-1 receptors, homotypic and heterotypic (TIR domain-containing adaptors) interactions |
| Mal | TLR4, MyD88, IRAK-2 and homotypic interactions |