For fluorescent western blotting, we highly recommend using the TrueBlack® Fluorescent Western Blot Blocking Buffer Kit #40683. This kit is specifically formulated to increase the specificity and sensitivity of the fluorescent western blot assay by blocking non-specific interaction between fluorophore-labeled antibodies and the western blotting membrane. This results in less background and brighter target signal when using the TrueBlack® Fluorescent Western Blot Blocking Buffer Kit compared to the standard antibody diluent and blocking buffers. If using this kit, please use the kit-specific protocol on the product webpage or the datasheet.
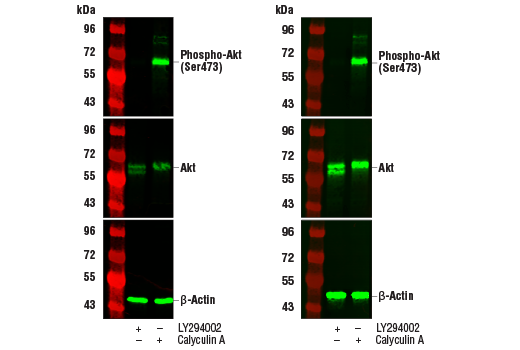
Western blot analysis of extracts from Jurkat cells, untreated (-) or treated with LY294002 #9901 or Calyculin A #9902 (+), using Phospho-Akt (Ser473) (D9E) XP® Rabbit mAb #4060 (upper), Akt (pan) (C67E7) Rabbit mAb #4691 (middle), or β-Actin (D6A8) Rabbit mAb #8457 (lower). The western blot membranes were treated with our standard antibody diluent and blocking buffers (left) or with the TrueBlack® Fluorescent Western Blot Blocking Buffer Kit (right). Increased antibody sensitivity and decreased background fluorescence can be seen when using the TrueBlack® Fluorescent Western Blot Blocking Buffer Kit.
NOTE: Two-color western blots require primary antibodies from different species and appropriate secondary antibodies labeled with different dyes. Overlap of epitopes may cause interference and should be considered in two color western blots. If the primary antibodies require different primary antibody incubation buffers, test each primary individually in both buffers to determine the optimal one for the dual-labeling experiment.
For western blots, incubate membrane with diluted primary antibody in either 5% w/v BSA or nonfat dry milk, 1X TBS, 0.1% Tween® 20 at 4°C with gentle shaking, overnight. Please refer to primary antibody product webpage for recommended primary antibody dilution buffer and recommended antibody dilution.
NOTE: Prepare solutions with reverse osmosis deionized (RODI) or equivalent grade water.
Load 20 µl onto SDS-PAGE gel (10 cm x 10 cm).
NOTE: Loading of prestained molecular weight markers (#59329, 10 µl/lane) is recommended to verify electrotransfer and to determine molecular weights. Prestained markers are autofluorescent at near-infrared wavelengths.
NOTE: Volumes are for 10 cm x 10 cm (100 cm2) of membrane; for different sized membranes, adjust volumes accordingly.
Incubate membrane in 25 ml of blocking buffer for 1 hr at room temperature.
CRITICAL STEP: Do not include Tween® 20 in blocking buffer (Section A, Step 8).
Drain membrane of excess TBST and allow to dry.
CRITICAL STEP: Membrane must be dry for fluorescent staining.
posted March 2022
revised March 2022