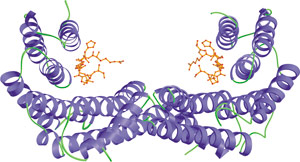
Human 14-3-3 ζ dimer bound to TrkA phospho-peptide (red).
14-3-3 genes encode an ubiquitous family of highly conserved acidic proteins of ~30 kDa that are found in eukaryotic lineages as diverse as fungi, plants and animals. Mammals encode seven closely related family members (β, γ, ε, η, σ, τ, and ζ) that are implicated in a wide variety of cellular processes including apoptosis, stress response and cell cycle. Their most notable function is their binding to phosphoserine/phosphothreonine motifs in a sequence-specific manner. The structure of 14-3-3 proteins have a tertiary structure consisting of nine α-helices for each monomer, with the biologically functional dimer resembling a “U” shaped structure. 14-3-3 proteins have been linked to a number of diseases such as cancer, Cruezfeldt-Jacob disease, Alzheimers, and Miller-Dieker syndrome.

| 14-3-3 Binding Partners | Functions |
| cdc25 tyrosine phosphatases | Cell cycle regulation |
| Bad Bcl-xL binding partner | Regulation of apoptosis |
| c-Raf Ser/Thr Kinase | Regulation of kinase activity; Signal transduction |
| PKC Ser/Thr Kinase | Signal transduction |
| MEKK1,2,3 Ser/Thr Kinase | Signal transduction |
R-X-[Ar/S]-[+]-pS-[LEAM]-P or R-[S/Ar]-[+]-pS-[LEAM]-P
Where Ar=aromatic residue, [+]=basic residue and pS=phosphoserine