CST signaling pathway diagrams allow you to click on individual nodes to find research resources or product information. You can also download the pathway diagrams for educational and research purposes.
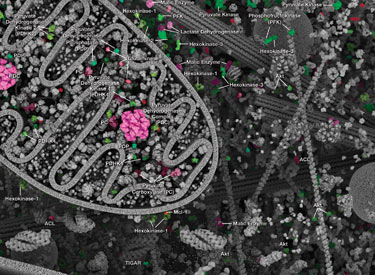
Glucose is the primary energy source for most cells of the body. The study of glucose metabolism is central to cell proliferation, growth, survival, and most recently, tumor progression. The maintenance of glucose homeostasis is an essential physiological process that is regulated by hormones. An elevation in blood glucose levels during feeding stimulates insulin release from pancreatic β cells through a glucose-sensing pathway. Insulin stimulates glucose uptake from blood into skeletal muscle and adipose tissue through a signaling cascade mediated by the insulin receptor (IR). Insulin binding to the IR results in activation of the insulin receptor substrate (IRS) protein and subsequent signaling to the PI3K/Akt and Erk1/2 pathways, resulting in translocation of Glut4 vesicles, glucose uptake, cell proliferation, and survival. Aberrant insulin signaling can result in diabetes and obesity, and perhaps atherosclerosis and even neurodegenerative disease.
During conditions of glucose deprivation when cellular ATP levels fall, the serine/threonine kinase AMPK becomes active. AMPK is an energy sensor that is activated by an elevated AMP/ATP ratio due to cellular and environmental stress, such as low glucose, heat shock, hypoxia, and ischemia. AMPK activation positively regulates signaling pathways that replenish cellular ATP supplies. For example, activation of AMPK enhances both the transcription and translocation of Glut4, resulting in an increase in insulin-stimulated glucose uptake. In addition, it also stimulates catabolic processes such as fatty acid oxidation and glycolysis via inhibition of ACC and activation of PFK2. AMPK negatively regulates several proteins central to ATP-consuming processes such as mTORC2, glycogen synthase, SREBP-1, and TSC2, resulting in the downregulation or inhibition of gluconeogenesis and glycogen, lipid, and protein synthesis.
Recent focus in the field centers on the metabolism of cancer. In the 1920s, Otto Warburg observed that tumor cells undergo high rates of glycolysis and lactate production in the presence of oxygen, a condition usually found only in the oxygen-deprived state. This phenomenon was later termed the Warburg Effect. Several signaling pathways such as Akt, Erk1/2, and AMPK converge on the key glycolytic enzymes PFK and PKM2 to regulate activity and direct production of ATP synthesis. The transcription factors c-Myc and p53 also play an important role in this process by regulating glutamine metabolism, lipid metabolism, and the pentose phosphate shunt, thereby creating the cellular components (nucleotides, lipids, proteins, Krebs Cycle intermediates) necessary for supporting and sustaining rapid tumor cell growth.