Revision 17
#29666
Store at +4C
FastScan™ Cas9 (S. pyogenes) ELISA Kit
1 x 96 tests
Species Cross Reactivity:
All
UniProt ID:
#Q99ZW2
Entrez-Gene Id:
#901176
877-616-CELL (2355)
877-678-TECH (8324)
3 Trask Lane | Danvers | Massachusetts | 01923 | USA
For Research Use Only. Not for Use in Diagnostic Procedures.
| Product Includes | Product # | Quantity | Color |
|---|---|---|---|
| FastScan™ ELISA Microwell Plate, 384 Well ("D" Kit Only) | 85242 | 384 tests | |
| FastScan™ ELISA Microwell Strip Plate, 96 Well | 53257 | 96 tests | |
| Cas9 (S. pyogenes) Rabbit Capture mAb | 75578 | 1 ea | Green (Lyophilized) |
| Cas9 Mouse HRP-linked mAb | 54725 | 1 ea | Red (Lyophilized) |
| FastScan™ ELISA Capture Antibody Diluent | 16076 | 3 ml | Green |
| FastScan™ ELISA HRP Antibody Diluent | 28120 | 3 ml | |
| TMB Substrate | 7004 | 11 ml | |
| STOP Solution | 7002 | 11 ml | |
| Sealing Tape | 54503 | 1 ea | |
| ELISA Wash Buffer (20X) | 9801 | 25 ml | |
| FastScan™ ELISA Cell Extraction Buffer (5X) | 69905 | 10 ml | |
| FastScan™ ELISA Cell Extraction Enhancer Solution (50X) | 25243 | 1 ml | |
| FastScan™ ELISA Kit #29666 Positive Control Type 2 | 93324 | 1 ea |
Kit contents scale proportionally with size, except sealing tape.
Example: The V1 kit contains 5X the listed quantities above, but will exclude the sealing tape.
For the “C” and “V” kits, the supplied 96-well strip plate consists of twelve 8-well strips in a support frame. This enables custom plate configurations.
For the “D” kits, the supplied 384-well microplate is a single piece of material and does not break apart.
Description
*Antibodies in kit are custom formulations specific to kit.
IMPORTANT: This FastScan™ ELISA Kit requires 4 washes at Step 6 of the protocol.
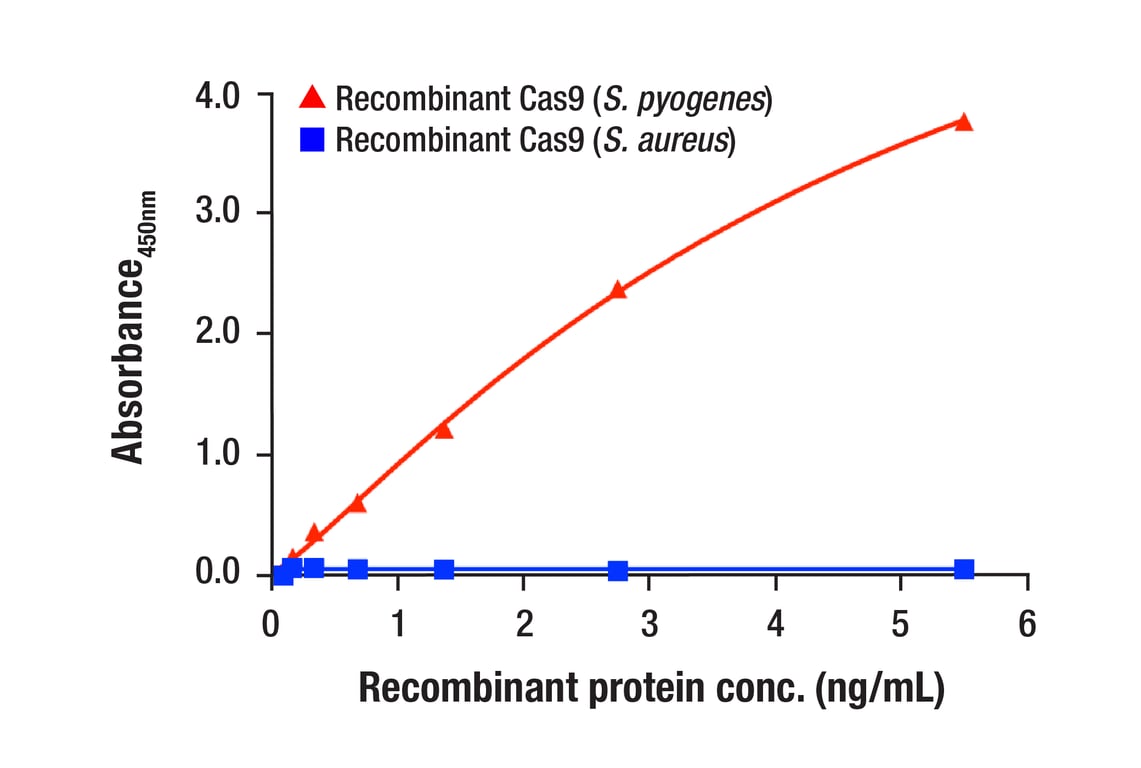
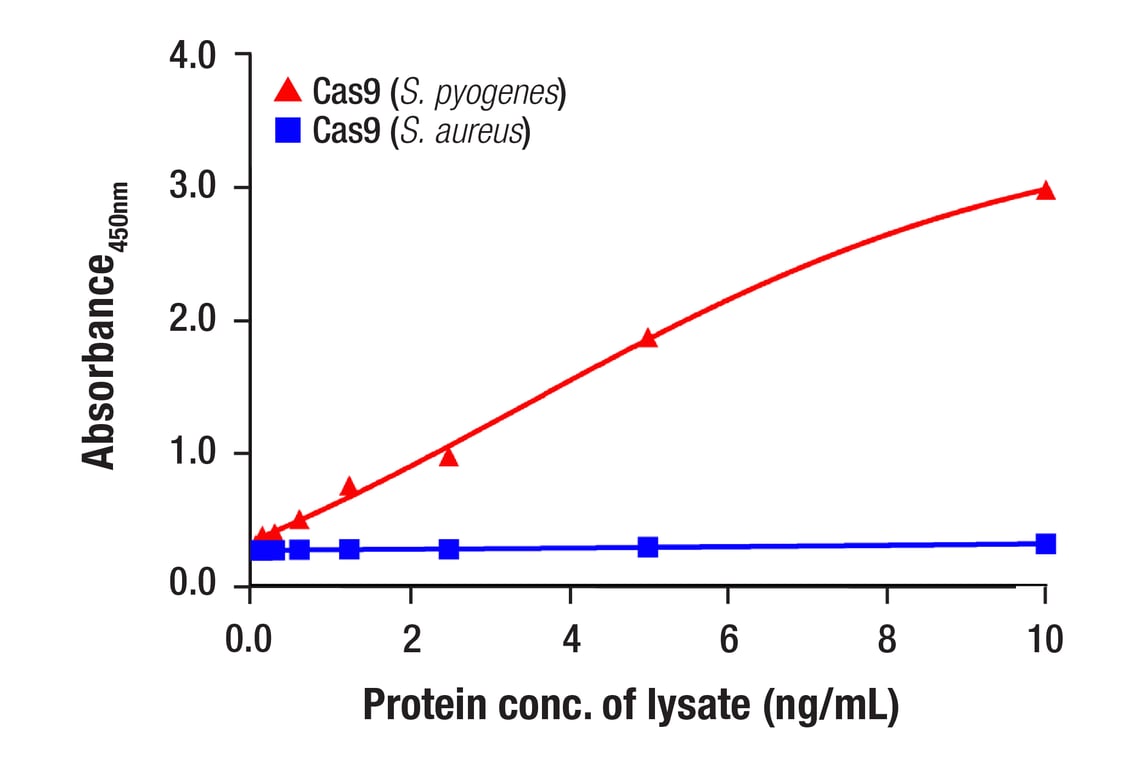
Specificity/Sensitivity
Background
Background References
- Horvath, P. and Barrangou, R. (2010) Science 327, 167-70.
- Wiedenheft, B. et al. (2012) Nature 482, 331-8.
- Singh, P. et al. (2015) Genetics 199, 1-15.
- Cong, L. et al. (2013) Science 339, 819-23.
- Mali, P. et al. (2013) Science 339, 823-6.
- Li, D. et al. (2013) Nat Biotechnol 31, 681-3.
- Shen, B. et al. (2013) Cell Res 23, 720-3.
- Niu, Y. et al. (2014) Cell 156, 836-43.
Trademarks and Patents
Cell Signaling Technology is a trademark of Cell Signaling Technology, Inc.
FastScan™ ELISA is a registered trademark of Cell Signaling Technology, Inc.
U.S. Patents 9,086,407, 9,261,500, and 9,476,874, foreign equivalents, and child patents deriving therefrom.
All other trademarks are the property of their respective owners. Visit cellsignal.com/trademarks for more information.
Limited Uses
Except as otherwise expressly agreed in a writing signed by a legally authorized representative of CST, the following terms apply to Products provided by CST, its affiliates or its distributors. Any Customer's terms and conditions that are in addition to, or different from, those contained herein, unless separately accepted in writing by a legally authorized representative of CST, are rejected and are of no force or effect.
Products are labeled with For Research Use Only or a similar labeling statement and have not been approved, cleared, or licensed by the FDA or other regulatory foreign or domestic entity, for any purpose. Customer shall not use any Product for any diagnostic or therapeutic purpose, or otherwise in any manner that conflicts with its labeling statement. Products sold or licensed by CST are provided for Customer as the end-user and solely for research and development uses. Any use of Product for diagnostic, prophylactic or therapeutic purposes, or any purchase of Product for resale (alone or as a component) or other commercial purpose, requires a separate license from CST. Customer shall (a) not sell, license, loan, donate or otherwise transfer or make available any Product to any third party, whether alone or in combination with other materials, or use the Products to manufacture any commercial products, (b) not copy, modify, reverse engineer, decompile, disassemble or otherwise attempt to discover the underlying structure or technology of the Products, or use the Products for the purpose of developing any products or services that would compete with CST products or services, (c) not alter or remove from the Products any trademarks, trade names, logos, patent or copyright notices or markings, (d) use the Products solely in accordance with CST Product Terms of Sale and any applicable documentation, and (e) comply with any license, terms of service or similar agreement with respect to any third party products or services used by Customer in connection with the Products.
Revision 17
Revision 17
FastScan™ ELISA Protocol
(Colorimetric 96 well)
A. Solutions and Reagents
NOTE: Prepare solutions with deionized/purified water or equivalent. Prepare only as much reagent as needed on the day of the experiment.
- FastScan™ ELISA Microwell Strip Plate, 96 well #53257: Before opening the sealed bag, allow it to reach room temperature. Unused microwell strips should be returned to the original resealable bag containing the desiccant pack and stored at 4°C.
- 1X ELISA Wash Buffer: Prepare by diluting ELISA Wash Buffer (20X) (included in each kit) to 1X with deionized water.
- 1X Cell Extraction Buffer: Prepare by diluting FastScan™ ELISA Cell Extraction Buffer (5X) #69905 and FastScan™ ELISA Cell Extraction Enhancer Solution (50X) #25243* to 1X with deionized water. This buffer can be stored at 4°C for short-term use (1-2 weeks). To make 10 mL 1X Cell Extraction Buffer, combine 7.8 mL deionized water, 2 mL FastScan™ ELISA Cell Extraction Buffer (5X), and 200 μL FastScan™ ELISA Cell Extraction Enhancer Solution (50X). Alternatively, Enhancer Solution may be added to the Cell Extraction Buffer after extraction of cells or tissue. When using the 1X Cell Extraction Buffer as a sample diluent for the assay, it is recommended to equilibrate it to room temperature prior to use.
*IMPORTANT: The provided FastScan™ ELISA Cell Extraction Enhancer Solution (50X) may precipitate when stored at 4°C. To dissolve, warm briefly at 37°C and mix gently. Store FastScan™ ELISA Cell Extraction Enhancer Solution (50X) at room temperature to avoid precipitation.
NOTE: The 1X Cell Extraction Buffer contains phosphatase inhibitors. Protease inhibitors should be added to the 1X Cell Extraction Buffer immediately prior to lysing cells. Additional phosphatase inhibitors can also be added (e.g., Protease/Phosphatase Inhibitor Cocktail (100X) #5872, not supplied).
- FastScan™ ELISA Capture Antibody Diluent: Green diluent for reconstitution of the Capture Antibody.
- FastScan™ ELISA HRP Antibody Diluent: Diluent (amber bottle) for reconstitution of the HRP-linked Antibody. Protect from light.
- 4X Capture Antibody: Reconstitute lyophilized Capture Antibody (green colored cake) with 3 mL FastScan™ ELISA Capture Antibody Diluent (green diluent). Incubate at room temperature for 5 min with occasional gentle mixing to fully reconstitute. For best results, use immediately following antibody reconstitution. Unused reconstituted 4X Capture Antibody may be stored for up to 4 weeks at 4°C, although there may be some loss of signal compared to freshly reconstituted antibody.
- 4X HRP-linked Antibody: Reconstitute lyophilized HRP-linked Antibody (red colored cake) with 3 mL FastScan™ ELISA HRP Antibody Diluent. Incubate at room temperature for 5 min with occasional gentle mixing to fully reconstitute. For best results, use immediately following antibody reconstitution. Unused reconstituted 4X HRP-linked Antibody may be stored for up to 4 weeks at 4°C protected from light, although there may be some loss of signal compared to freshly reconstituted antibody.
- Antibody Cocktail: Combine equal volumes of the reconstituted 4X Capture and 4X HRP-linked Antibodies immediately prior to assay and mix. To make 6 mL of the Antibody Cocktail (enough for 1X 96-well plate), combine 3 mL 4X Capture Antibody with 3 mL 4X HRP-linked Antibody.
-
Positive Control: Reconstitute 1 vial of lyophilized Positive
Control (refer to product datasheet or vial label to determine which
type of Positive Control is included with the kit):
- For Positive Control Type 1, add 250 μL deionized water.
- For Positive Control Type 2, add 500 μL 1X Cell Extraction Buffer.
NOTE: A select number of FastScan™ ELISA kits do not contain a positive control. Please refer to the Product Includes table on the product webpage or datasheet for a list of included reagents. Should you need support on how to generate a positive control for those kits, contact CST technical support at [email protected].
- TMB Substrate #7004: Bring to room temperature before use.
- STOP Solution #7002: Bring to room temperature before use.
B. Preparing Cell Lysates
For Adherent Cells
- Aspirate media when the culture reaches 80-90% confluence.
- Remove media and rinse cells once with ice-cold 1X PBS.
- Remove PBS, add 0.5 mL ice-cold 1X Cell Extraction Buffer (recommended to supplement with protease inhibitors and additional phosphatase inhibitors as needed) to each plate (10 cm diameter), and incubate the plate on ice for 5 min.
- Scrape cells off the plate and transfer to an appropriate tube. Keep on ice.
- Sonicate lysates on ice.
- Microcentrifuge for 5 min (x14,000 rpm) at 4°C and transfer the supernatant to a new tube. The supernatant is the cell lysate. Store at −80°C in single-use aliquots.
For Suspension Cells
- Remove media by low speed centrifugation (~1200 rpm) when the culture reaches 0.5-1.0 x 106 viable cells/mL.
- Wash once with ice-cold 1X PBS.
- Cells harvested from 50 mL of growth media can be lysed in 2.0 mL of 1X Cell Extraction Buffer (recommended to supplement with protease inhibitors and additional phosphatase inhibitors as needed).
- Sonicate lysates on ice.
- Microcentrifuge for 5 min (x14,000 rpm) at 4°C and transfer the supernatant to a new tube. The supernatant is the cell lysate. Store at −80°C in single-use aliquots.
C. Test Procedure
NOTE: Equilibrate all materials and prepared reagents to room temperature prior to running the assay.
- Prepare all reagents as indicated above (Section A).
- Samples should be undiluted or diluted with 1X Cell Extraction Buffer to a 2X protein concentration to achieve a final 1X protein concentration upon addition of the antibody cocktail. Individual datasheets for each kit provide a sensitivity curve that serves as a reference for selection of an appropriate starting lysate concentration. The sensitivity curve shows typical results across a range of lysate concentration points.
- Add 50 μL of each sample or Positive Control to the appropriate wells.
- Add 50 μL of the Antibody Cocktail to each well.
- Seal the plate with the supplied sealing tape and incubate for 1 hr at room temperature on a plate shaker set to 400 rpm (moderate agitation).
-
Gently remove the tape and wash wells:
- Discard plate contents into a receptacle.
- Wash 3 times* with 1X ELISA Wash Buffer, 200 μL each time for every well. After each wash, aspirate or decant from wells. Invert the plate and blot it against clean paper towels to remove the residual solution in each well, but do not allow wells to completely dry at any time.
- Clean the underside of all wells with a lint-free tissue.
*NOTE: Certain FastScan™ ELISA Kits may require additional washes at this step. Any requirements for additional washes will be specifically noted in the “Product Description” on the product webpage or datasheet.
- Add 100 μL of TMB Substrate to each well. Seal with tape and incubate the plate in the dark for 15 min at room temperature on a plate shaker (400 rpm, moderate agitation) or for 10 min at 37°C without shaking.
- Add 100 μL of STOP Solution to each well. Shake gently for a few seconds.
NOTE: Initial color of positive reaction is blue, which changes to yellow upon addition of STOP Solution.
-
Read results:
- Visual Determination: Read within 30 min after adding STOP Solution.
- Spectrophotometric Determination: Clean the underside of all wells with a lint-free tissue. Read absorbance at 450 nm within 30 min after adding STOP Solution.
FastScan™ ELISA Protocol
(Colorimetric 384 well)
A. Solutions and Reagents
NOTE: Prepare solutions with deionized/purified water or equivalent. Prepare only as much reagent as needed on the day of the experiment.
- FastScan™ ELISA Microwell Plate, 384 well #85242: Before opening the sealed bag, allow it to reach room temperature.
- 1X ELISA Wash Buffer: Prepare by diluting ELISA Wash Buffer (20X) (included in each kit) to 1X with deionized water.
- 1X Cell Extraction Buffer: Prepare by diluting FastScan™ ELISA Cell Extraction Buffer (5X) #69905 and FastScan™ ELISA Cell Extraction Enhancer Solution (50X) #25243* to 1X with deionized water. This buffer can be stored at 4°C for short-term use (1-2 weeks). To make 10 mL 1X Cell Extraction Buffer, combine 7.8 mL deionized water, 2 mL FastScan™ ELISA Cell Extraction Buffer (5X), and 200 μL FastScan™ ELISA Cell Extraction Enhancer Solution (50X). Alternatively, Enhancer Solution may be added to the Cell Extraction Buffer after extraction of cells or tissue. When using the 1X Cell Extraction Buffer as a sample diluent for the assay, it is recommended to equilibrate it to room temperature prior to use.
*IMPORTANT: The provided FastScan™ ELISA Cell Extraction Enhancer Solution (50X) may precipitate when stored at 4°C. To dissolve, warm briefly at 37°C and mix gently. Store FastScan™ ELISA Cell Extraction Enhancer Solution (50X) at room temperature to avoid precipitation.
NOTE: The 1X Cell Extraction Buffer contains phosphatase inhibitors. Protease inhibitors should be added to the 1X Cell Extraction Buffer immediately prior to lysing cells. Additional phosphatase inhibitors can also be added (e.g., Protease/Phosphatase Inhibitor Cocktail (100X) #5872, not supplied).
- FastScan™ ELISA Capture Antibody Diluent: Green diluent for reconstitution of the Capture Antibody.
- FastScan™ ELISA HRP Antibody Diluent: Diluent (amber bottle) for reconstitution of the HRP-linked Antibody. Protect from light.
- 4X Capture Antibody: Reconstitute lyophilized Capture Antibody (green colored cake) with 3 mL FastScan™ ELISA Capture Antibody Diluent (green diluent). Incubate at room temperature for 5 min with occasional gentle mixing to fully reconstitute. For best results, use immediately following antibody reconstitution. Unused reconstituted 4X Capture Antibody may be stored for up to 4 weeks at 4°C, although there may be some loss of signal compared to freshly reconstituted antibody.
- 4X HRP-linked Antibody: Reconstitute lyophilized HRP-linked Antibody (red colored cake) with 3 mL FastScan™ ELISA HRP Antibody Diluent. Incubate at room temperature for 5 min with occasional gentle mixing to fully reconstitute. For best results, use immediately following antibody reconstitution. Unused reconstituted 4X HRP-linked Antibody may be stored for up to 4 weeks at 4°C protected from light, although there may be some loss of signal compared to freshly reconstituted antibody.
- Antibody Cocktail: Combine equal volumes of the reconstituted 4X Capture and 4X HRP-linked Antibodies immediately prior to assay and mix. To make 6 mL of the Antibody Cocktail (enough for 1X 384-well plate), combine 3 mL 4X Capture Antibody with 3 mL 4X HRP-linked Antibody.
-
Positive Control: Reconstitute 1 vial of lyophilized Positive
Control (refer to product datasheet or vial label to determine which
type of Positive Control is included with the kit):
- For Positive Control Type 1, add 250 μL deionized water.
- For Positive Control Type 2, add 500 μL 1X Cell Extraction Buffer.
- TMB Substrate #7004: Bring to room temperature before use.
- STOP Solution #7002: Bring to room temperature before use
NOTE: A select number of FastScan™ ELISA kits do not contain a positive control. Please refer to the Product Includes table on the product webpage or datasheet for a list of included reagents. Should you need support on how to generate a positive control for those kits, contact CST technical support at [email protected].
B. Preparing Cell Lysates
For Adherent Cells
- Aspirate media when the culture reaches 80–90% confluence.
- Remove media and rinse cells once with ice-cold 1X PBS.
- Remove PBS, add 0.5 mL ice-cold 1X Cell Extraction Buffer (recommended to supplement with protease inhibitors and additional phosphatase inhibitors as needed) to each plate (10 cm diameter), and incubate the plate on ice for 5 min.
- Scrape cells off the plate and transfer to an appropriate tube. Keep on ice.
- Sonicate lysates on ice.
- Microcentrifuge for 5 min (x14,000 rpm) at 4°C and transfer the supernatant to a new tube. The supernatant is the cell lysate. Store at −80°C in single-use aliquots.
For Suspension Cells
- Remove media by low speed centrifugation (~1200 rpm) when the culture reaches 0.5–1.0 x 106 viable cells/mL.
- Wash once with ice-cold 1X PBS.
- Cells harvested from 50 mL of growth media can be lysed in 2.0 mL of 1X Cell Extraction Buffer (recommended to supplement with protease inhibitors and additional phosphatase inhibitors as needed).
- Sonicate lysates on ice.
- Microcentrifuge for 5 min (x14,000 rpm) at 4°C and transfer the supernatant to a new tube. The supernatant is the cell lysate. Store at −80°C in single-use aliquots.
C. Test Procedure
NOTE: Equilibrate all materials and prepared reagents to room temperature prior to running the assay.
- Prepare all reagents as indicated above (Section A).
- Samples should be undiluted or diluted with 1X Cell Extraction Buffer to a 2X protein concentration to achieve a final 1X protein concentration upon addition of the antibody cocktail. Individual datasheets for each kit provide a sensitivity curve that serves as a reference for selection of an appropriate starting lysate concentration. The sensitivity curve shows typical results across a range of lysate concentration points.
- Add 12.5 μL of each sample or Positive Control to the appropriate wells.
- Add 12.5 μL of the Antibody Cocktail to each well.
- Seal the plate with the supplied sealing tape and incubate for 1 hr at room temperature on a plate shaker set to 400 rpm (moderate agitation). If increased signal is desired, increasing incubation time to 2 hr can increase signal for some kits.
-
Gently remove the tape and wash wells:
- Discard plate contents into a receptacle.
- Wash 4 times with 1X ELISA Wash Buffer, 100 μL each time for every well. After each wash, aspirate or decant from wells. Invert the plate and blot it against clean paper towels to remove the residual solution in each well, but do not allow wells to completely dry at any time.
- Clean the underside of all wells with a lint-free tissue.
- Add 25 μL of TMB Substrate to each well. Seal with tape and incubate the plate in the dark for 15 min at room temperature on a plate shaker (400 rpm, moderate agitation) or for 10 min at 37°C without shaking. If increased signal is desired, increasing TMB incubation time at 37°C can increase signal for some kits; however, there may also be an increase in blank signal.
- Add 25 μL of STOP Solution to each well. Shake gently for a few seconds.
NOTE: Initial color of positive reaction is blue, which changes to yellow upon addition of STOP Solution.
-
Read results:
- Visual Determination: Read within 30 min after adding STOP Solution.
- Spectrophotometric Determination: Clean the underside of all wells with a lint-free tissue. Read absorbance at 450 nm within 30 min after adding STOP Solution.
For Research Use Only. Not for Use in Diagnostic Procedures.
posted August 2025