Revision 6
#88005
Store at +4C
SARS-CoV-2 Spike Protein Multi-Domain (S1-NTD, RBD, S1, S2) Serological IgG ELISA Kit
Species Cross Reactivity:
H
UniProt ID:
#P0DTC2
Entrez-Gene Id:
#43740568
877-616-CELL (2355)
877-678-TECH (8324)
3 Trask Lane | Danvers | Massachusetts | 01923 | USA
For Research Use Only. Not for Use in Diagnostic Procedures.
| Product Includes | Product # | Quantity | Color | Storage Temp |
|---|---|---|---|---|
| Spike Multi-Domain Protein Coated Microwells | 43037 | 96 tests | +4C | |
| Anti-Human IgG, HRP-linked Antibody (ELISA Formulated) | 94210 | 1 ea | Red (Lyophilized) | +4C |
| Sample Diluent A | 71637 | 25 ml | +4C | |
| HRP Diluent | 13515 | 11 ml | Red | +4C |
| ELISA Wash Buffer (20X) | 9801 | 25 ml | +4C | |
| TMB Substrate | 7004 | 11 ml | +4C | |
| STOP Solution | 7002 | 11 ml | +4C | |
| Sealing Tape | 54503 | 2 ea | +4C | |
| ELISA Kit #88005 Positive Control | 57510 | 1 ea | +4C | |
| ELISA Kit #88005 Negative Control | 73072 | 1 ea | +4C |
Kit contents scale proportionally with size, except sealing tape.
Example: The V1 kit contains 5X the listed quantities above, but will exclude the sealing tape.
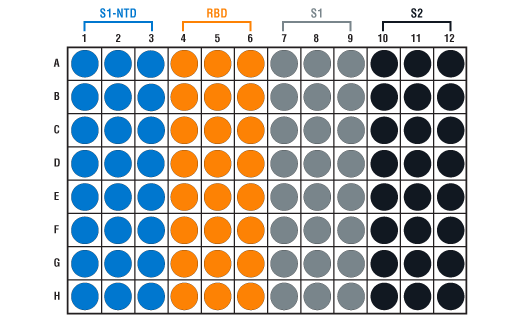
For the “C” and “V” kits, the supplied 96-well strip plate consists of twelve 8-well strips in a support frame. This enables custom plate configurations.
Description
*Antibodies in this kit are custom formulations specific to kit.
Specificity/Sensitivity
Background
Background References
- Zhou, P. et al. (2020) Nature 579, 270-3.
- Tortorici, M.A. and Veesler, D. (2019) Adv Virus Res 105, 93-116.
- Li, F. et al. (2006) J Virol 80, 6794-800.
- Li, F. (2016) Annu Rev Virol 3, 237-61.
- Shang, J. et al. (2020) Nature 581, 221-4.
- Wrapp, D. et al. (2020) Science 367, 1260-3.
- Yan, R. et al. (2020) Science 367, 1444-8.
- Yuan, Y. et al. (2017) Nat Commun 8, 15092.
- Amanat, F. and Krammer, F. (2020) Immunity 52, 583-9.
Trademarks and Patents
Cell Signaling Technology is a trademark of Cell Signaling Technology, Inc.
All other trademarks are the property of their respective owners. Visit cellsignal.com/trademarks for more information.
Limited Uses
Except as otherwise expressly agreed in a writing signed by a legally authorized representative of CST, the following terms apply to Products provided by CST, its affiliates or its distributors. Any Customer's terms and conditions that are in addition to, or different from, those contained herein, unless separately accepted in writing by a legally authorized representative of CST, are rejected and are of no force or effect.
Products are labeled with For Research Use Only or a similar labeling statement and have not been approved, cleared, or licensed by the FDA or other regulatory foreign or domestic entity, for any purpose. Customer shall not use any Product for any diagnostic or therapeutic purpose, or otherwise in any manner that conflicts with its labeling statement. Products sold or licensed by CST are provided for Customer as the end-user and solely for research and development uses. Any use of Product for diagnostic, prophylactic or therapeutic purposes, or any purchase of Product for resale (alone or as a component) or other commercial purpose, requires a separate license from CST. Customer shall (a) not sell, license, loan, donate or otherwise transfer or make available any Product to any third party, whether alone or in combination with other materials, or use the Products to manufacture any commercial products, (b) not copy, modify, reverse engineer, decompile, disassemble or otherwise attempt to discover the underlying structure or technology of the Products, or use the Products for the purpose of developing any products or services that would compete with CST products or services, (c) not alter or remove from the Products any trademarks, trade names, logos, patent or copyright notices or markings, (d) use the Products solely in accordance with CST Product Terms of Sale and any applicable documentation, and (e) comply with any license, terms of service or similar agreement with respect to any third party products or services used by Customer in connection with the Products.
Revision 6
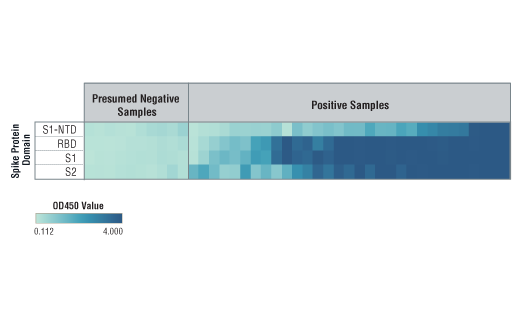
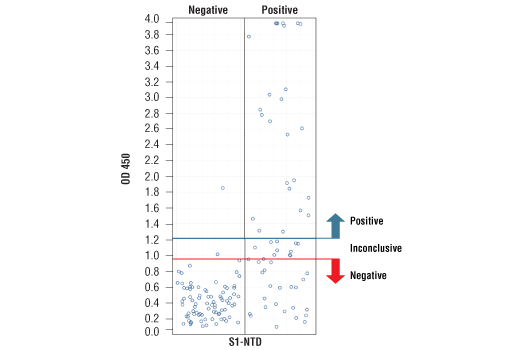
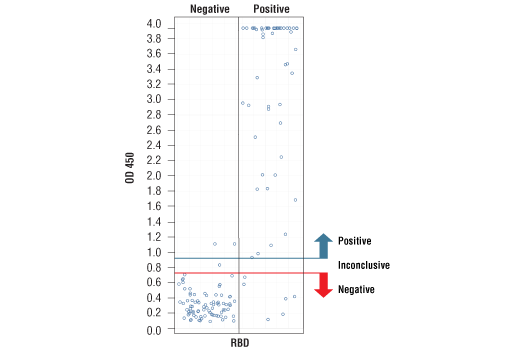
*Positive reference samples were from patient donors with a positive SARS-CoV-2 diagnosis. However, a positive SARS-CoV-2 diagnosis will not always correlate with a positive response aganst each spike domain protein in this IgG Serological ELISA as differences in disease severity, timing of sample collection relative to disease onset, and patient profiles may affect presence and abundance of antibodies against each spike domain in the reference sample.
Note: We are continuing to test more samples as available. For the most up-to-date set of data, always refer to the product page for #88005 on the website.
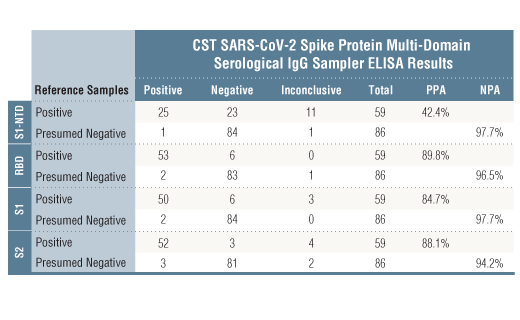
*The included Positive Control in the kit is intended to give a positive signal for the RBD and S1 coated microwell strips, and is NOT a Positive Control for the S1-NTD or S2 microwells.
Revision 6
*Positive reference samples were from patient donors with a positive SARS-CoV-2 diagnosis. However, a positive SARS-CoV-2 diagnosis will not always correlate with a positive response against each spike domain protein in this IgG Serological ELISA as differences in disease severity, timing of sample collection relative to disease onset, and patient profiles may affect presence and abundance of antibodies against each spike domain in the reference sample.
*Positive reference samples were from patient donors with a positive SARS-CoV-2 diagnosis. However, a positive SARS-CoV-2 diagnosis will not always correlate with a positive response against each spike domain protein in this IgG Serological ELISA as differences in disease severity, timing of sample collection relative to disease onset, and patient profiles may affect presence and abundance of antibodies against each spike domain in the reference sample.
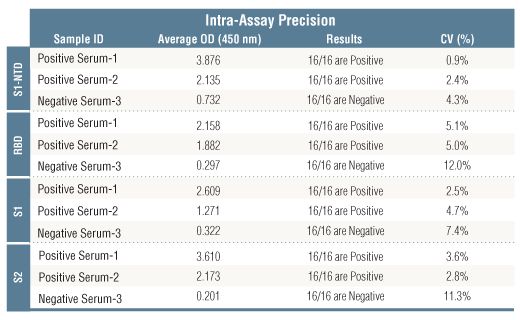
Revision 6
*Positive reference samples were from patient donors with a positive SARS-CoV-2 diagnosis. However, a positive SARS-CoV-2 diagnosis will not always correlate with a positive response against each spike domain protein in this IgG Serological ELISA as differences in disease severity, timing of sample collection relative to disease onset, and patient profiles may affect presence and abundance of antibodies against each spike domain in the reference sample.
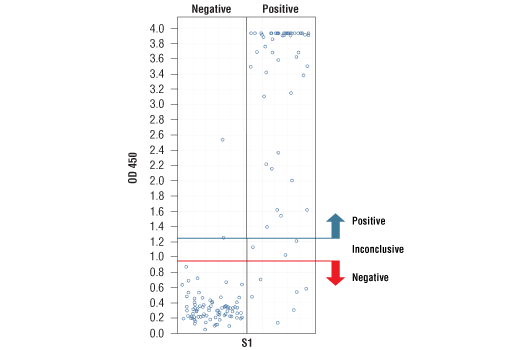
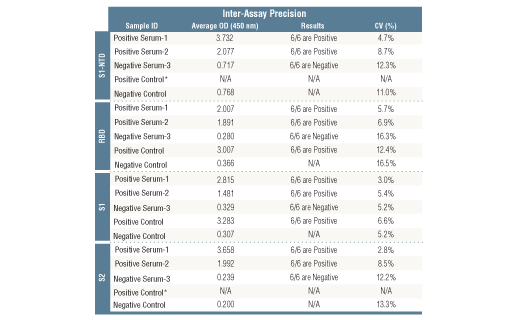
*Positive reference samples were from patient donors with a positive SARS-CoV-2 diagnosis. However, a positive SARS-CoV-2 diagnosis will not always correlate with a positive response against each spike domain protein in this IgG Serological ELISA as differences in disease severity, timing of sample collection relative to disease onset, and patient profiles may affect presence and abundance of antibodies against each spike domain in the reference sample.
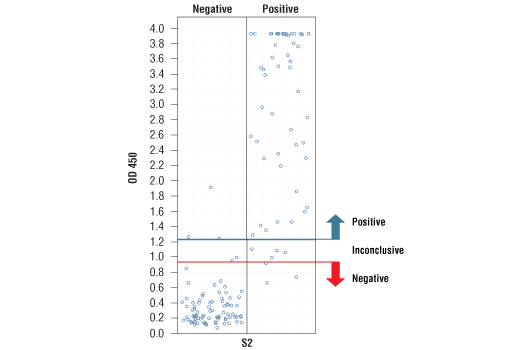
NOTE: Absorbance 450 nm values should not be directly compared across different spike domain proteins, as they are not absolute values.
*Positive reference samples were from patient donors with a positive SARS-CoV-2 diagnosis. However, a positive SARS-CoV-2 diagnosis will not always correlate with a positive response against each spike domain protein in this IgG Serological ELISA as differences in disease severity, timing of sample collection relative to disease onset, and patient profiles may affect presence and abundance of antibodies against each spike domain in the reference sample.