Revision 2
#93613
Store at -20C
877-616-CELL (2355)
877-678-TECH (8324)
3 Trask Lane | Danvers | Massachusetts | 01923 | USA
For Research Use Only. Not for Use in Diagnostic Procedures.
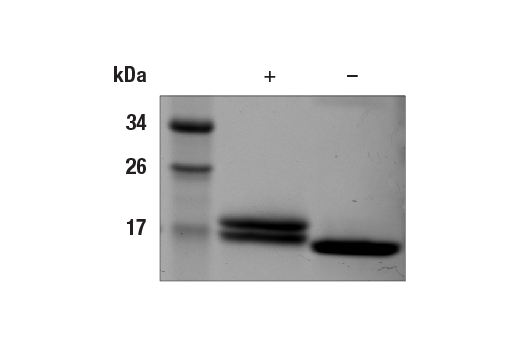
MW (kDa):
19.4
UniProt ID:
#P01563
Entrez-Gene Id:
3440
Background
The human IFN-α 2 subvariants 2a and 2b differ by one or two amino acids at positions 23 and/or 34 of the mature protein (4). Pegylated forms of IFN-α 2 and recombinant IFN-α 2 protein are used clinically as standard treatments for anti-viral and anti-tumor therapies (5). IFN-α 2a and 2b proteins have also been shown to have implications in treating hepatitis C (6).
Endotoxin
Purity
Source / Purification
Bioactivity
Background References
- Stiehm, E.R. et al. (1982) Ann Intern Med 96, 80-93.
- Pellegrini, S. et al. (1989) Mol Cell Biol 9, 4605-12.
- Pfeffer, L.M. and Colamonici, O.R. (1991) Pharmacol Ther 52, 149-57.
- von Gabain, A. et al. (1990) Eur J Biochem 190, 257-61.
- CA Cancer J Clin (1988) 38, 280-90.
- Castelruiz, Y. et al. (1999) Hepatology 29, 1900-4.
Trademarks and Patents
Cell Signaling Technology is a trademark of Cell Signaling Technology, Inc.
All other trademarks are the property of their respective owners. Visit cellsignal.com/trademarks for more information.
Limited Uses
Except as otherwise expressly agreed in a writing signed by a legally authorized representative of CST, the following terms apply to Products provided by CST, its affiliates or its distributors. Any Customer's terms and conditions that are in addition to, or different from, those contained herein, unless separately accepted in writing by a legally authorized representative of CST, are rejected and are of no force or effect.
Products are labeled with For Research Use Only or a similar labeling statement and have not been approved, cleared, or licensed by the FDA or other regulatory foreign or domestic entity, for any purpose. Customer shall not use any Product for any diagnostic or therapeutic purpose, or otherwise in any manner that conflicts with its labeling statement. Products sold or licensed by CST are provided for Customer as the end-user and solely for research and development uses. Any use of Product for diagnostic, prophylactic or therapeutic purposes, or any purchase of Product for resale (alone or as a component) or other commercial purpose, requires a separate license from CST. Customer shall (a) not sell, license, loan, donate or otherwise transfer or make available any Product to any third party, whether alone or in combination with other materials, or use the Products to manufacture any commercial products, (b) not copy, modify, reverse engineer, decompile, disassemble or otherwise attempt to discover the underlying structure or technology of the Products, or use the Products for the purpose of developing any products or services that would compete with CST products or services, (c) not alter or remove from the Products any trademarks, trade names, logos, patent or copyright notices or markings, (d) use the Products solely in accordance with CST Product Terms of Sale and any applicable documentation, and (e) comply with any license, terms of service or similar agreement with respect to any third party products or services used by Customer in connection with the Products.
Revision 2