The immune system is composed of tissues, cells, and molecules whose primary function is to detect, respond to, and eliminate pathogens and transformed cells.
There are two main components of the immune system—1) the innate immune system and 2) the adaptive immune system. The innate immune system acts as the first line of defense and detects pathogens via germ line-encoded pattern recognition receptors, does not form memory, and responds quickly (within minutes to hours). The adaptive immune system responds slowly (over days), uses antigen-specific receptors that go through multiple gene rearrangements during development, and forms immunologic memory—“learning” from the initial insult and readying the body to fight future exposure to similar harmful material. Both types of immune responses can either be driven by macromolecules within the extracellular fluid or by the activation of specific immune cells. These responses are known as humoral immunity and cell-mediated immunity, respectively.
Under the cell-mediated immune response umbrella, immune cells mostly stratify based on whether the response is innate or adaptive. The innate immune system mainly consists of phagocytic cells, such as neutrophils, macrophages, natural killer cells, basophils, and others that target dangerous invading organisms. The cells of the adaptive immune system are T cells and B cells. Natural killer (NK) T cells have features of both innate and adaptive immune cells. In addition, most other cell types, not specific to the immune system, can harbor intrinsic innate immune functions in the form of cytoplasmic receptors and signaling and effector molecules such as members of the RIG-I, STING and NLR families. As we will review further, cells of both systems arise from different myeloid and lymphoid lineages of hematopoietic stem cells.
Mature immune cells differentiate from what is known as hematopoietic stem cells. These are pluripotent undifferentiated progenitor cells found in the bone marrow, peripheral blood, and placenta. These hematopoietic stem cells can differentiate into the common myeloid progenitor cell or the common lymphoid progenitor cell.
Common lymphoid progenitor cells can differentiate into four major populations of lymphocytes that can be distinguished by cell-surface receptor expression: T cells, B cells, natural killer (NK) cells, and NK-T cells.
T cells play a critical role in cell-mediated immunity. These cells are identified by the expression of CD3 T cell signaling chains.
T cells arise from lymphoid progenitor cells that originally develop from hematopoietic stem cells in the bone marrow. Once the lymphoid progenitor commits to becoming a T cell, it migrates from the bone marrow to the thymus (hence the name T cell). The thymus provides the proper microenvironment where T cells can develop into their various subtypes, defined by the specific receptors expressed on the cell surface. Maturing T cells in the early stages, or thymocytes, lack receptors for both CD4 and CD8 and are thus called double negative (DN) cells. The DN cells then undergo genetic rearrangements and mutations to genes encoding for the alpha and beta T cell receptors (TCR), which starts them down a path of lineage commitment whereby the end result is a T cell receptor with specific affinity for a unique antigen.
The thymocytes are then selected for, both positively and negatively, to assure they recognize “foreign” antigens but not host antigens, which are presented as peptides via the MHC on the cell surface. Only cells that pass both tests will then be triggered to express CD4 and CD8, becoming double-positive cells. At the end of maturation, T cells will either express CD4 or CD8 (and lose expression of the other) and thus become single-positive cells. These mature CD4 or CD8-positive T cells are then released into the bloodstream and are initially in the naïve state, meaning they have not committed to a particular T cell subtype yet.
If the maturing thymocyte shows an intermediate amount of affinity for self MHC peptides during maturation then it may be selected to become a regulatory T cell (Treg) for its ability to recognize harmful “self” antigens. T cells in the blood or at peripheral tissues can also be locally induced to become Tregs. The main job of Tregs is to maintain tolerance to self-antigens, as well as limit T-effector cell function and proliferation.
Once arriving in the blood or peripheral tissue, naïve T cells can be induced to become a T-helper cell. Antigen-presenting cells (APCs), such as macrophages, dendritic cells, and B cells, will show the antigen to the T cell, and if its receptor has affinity the T cell will recognize the antigen. Typically, CD4+ T cells are suited to recognize peptide antigens bound to class II MHC proteins. Once the peptide-MHC complex is recognized, the T cell will activate a series of internal pathways that allow for the antigen recognition to be verified. Only then will the T-helper cell proliferate, expanding the pool of available cells that are specific for the harmful antigen, such as different bacteria and parasites.
Once T-helper cells have recognized their antigen and become activated, they will release a variety of cytokines, which are molecules that signal to other immune cells how to appropriately respond to the harmful molecule. There are several subsets of helper T cells including Th1, Th2 and Th17. These subsets produce and secrete distinct cytokines that help tailor the immune response depending on the type of pathogen. For instance, Th1 cells rely on the effector cytokine IFN-γ to signal macrophages to digest and destroy the harmful material, while Th2 cells secrete a combination of IL-4, IL-5, IL-9, IL-10, and IL-13 to trigger basophils, eosinophils, and other cells to attack the infection. The specific amount and varieties of cytokines will determine the appropriate response to the infection or harmful cell. T cells can also respond to cells of the self by becoming cytotoxic T cells (or natural killer T cells). Typically these are CD8+ cells that recognize virus-infected cells or tumor cells. Natural killer T cells (NKTs) will be discussed in more detail in a later section. These and other types of T cells perform their various duties or effector functions in disparate ways to recognize harmful material and recruit other immune cells to destroy it, as well as build a lasting immune response to the same type of insult.
The T cell receptor signaling interactive pathway is reviewed in detail here.
B cells are the critical cells in the blood that mediate antibody production. When harmful material is present in the blood stream, it is recognized as an antigen by specific receptors on the B cells. Then, after processing the antigen, and with the help of T cells, the B cell matures into plasma cells that secrete antibodies. B cells can also play a role in presenting antigens and cytokine secretion. Both the development and various functions of B cell will be discussed.
15% of leukocytes found in peripheral blood are B cells. B cells produce immunoglobulins (Ig), which are antigen binding proteins (also known as antibodies) comprised of two identical heavy chain and two identical lights chains. These immunoglobulins contain specific antigen biding sites that vary in amino acid sequence from one antibody molecule to another. These sites confer specificity of each antibody for recognition of a distinct antigen.
As mentioned above, B cells differentiate from hematopoietic cells found in the bone marrow. It is within the bone marrow that surface Ig receptors (antibodies) are assembled through a process of gene rearrangement. B cells utilize somatic joining of different genes on a multitude of chromosomes that encode the different parts of the heavy and light chains that make up the Ig. B cells are pushed toward differentiation through the production of IL-7 by bone marrow stromal cells. These developing B cells follow a program of sequential heavy and light chain rearrangements mediated by differential cell-surface receptor expression. For example, expression of a specific pre-B cell receptor prevents certain gene rearrangements on unrelated chromosomes to ensure that the mature B cell can express a certain Ig surface receptor capable of specifically recognizing a single antigen. This process is called allelic exclusion. Incorrect assembly of the Ig heavy chains and light chains can induce secondary rearrangement for receptor editing.
Aside from the cell-surface expression of an Ig receptor, B cells also contain transmembrane proteins that can induce intracellular signals after an antigen binds to the extracellular portion of the Ig receptor. B cell use similar intracellular signaling cascades to T cells that induce transcriptional changes leading to proliferation and maturation. Specifically, the intracellular domains of the Ig receptor can be phosphorylated by the src-family of kinases, initiating a cascade that is passed on by the tyrosine kinase Syk and a linker protein known as BLINK to phospholipase C and guanine exchange factors, ultimately activating protein kinase C, mobilizing calcium, and activating MAP kinase in a Ras/Rac-dependent manner.
A unique feature of B cells is their ability to undergo isotype switching. This specifically refers to a process of DNA rearrangement that can generate slightly different heavy chains. Additionally, alternative splicing of heavy chain exons can permit the Ig to remain cell-surface bound or to become secreted if the transmembrane exon is spliced out. Alternative splicing and isotype switching are induced through T cell derived cytokines. Additionally, T cells can induce somatic mutations that lead to changes within the antigen-binding sites of the Ig of the B cells. A self-correcting B cell function can detect whether these seemingly random mutations lead to a loss of affinity for the antigen and initiate a program for cell-death. Thus, antigens that activate both T cells and B cells trigger T cell mediated B cell maturation through isotype switching and somatic mutations, both of which are highly associated with B cell memory.
Memory responses are characterized by a rapid and extensive high-affinity Ig production to such antigens. The development of these memory responses are critical to the success of vaccination. However, they can also increase autoimmunity and allergic reactions.
T cell independent B cell activation by an antigen can also take place; however, this type of activation is linked to weak immune memory. Additionally, these antigens must be polymeric in order to activate B cells.
NB: Cytokines, or interleukins (ILs), are secreted molecules that act on transmembrane cell-surface receptors. Upon binding, these cytokines activate intracellular signal transduction pathways typically dependent on Jak-STAT signaling.
The B cell receptor signaling interactive pathway is reviewed in detail here.
Natural killer, or NK, cells are another cell type derived from the lymphoid lineage. Similar to B cells, they develop in the bone marrow in a cytokine and bone marrow stromal cell-dependent manner. They represent a small proportion of the peripheral blood and have no antigen-specific receptors. Instead, NK cells rely on a complex set of transmembrane receptors. In particular, they contain inhibitory cell-surface receptors that recognize class I HLA molecules and are thus inhibited by self-MHC molecules, killing only cells that have downregulated class I HLA expression. Therefore, they serve the important purpose of targeting virally-infected cells and tumor cells whose class I HLA expression is downregulated to evade death by CD8+ natural killer T (NK-T) cells. NK cells can also be activated in an antibody-, interferon-, or cytokine-dependent manner and, therefore, can play a large role in combating tumors. The mechanism of action of NK cells relies on the release of small granules containing granzymes and perforin from their cytoplasm. Upon their release these proteins can make pores and break down intracellular proteins in order to induce apoptosis. As such, NK cells are said to be cytotoxic.
NK-T cells are a special subtype of T cells that express certain receptors and markers typically expressed by NK cells in combination with a limited repertoire of T cell receptors. Therefore, this unique subtype shares properties of both T cells and NK cells. Unlike NK cells, which develop in the marrow, NK-T cells develop in the thymus.
Common myeloid progenitor cells, which are descendants of hematopoietic stem cells, give rise to several different types of myeloid cells, including macrophages, dendritic cells, and several other innate immune cells and blood cells.
Macrophages can be triggered to recognize antigens, such as damaged cells or foreign material, for on-demand destruction. Macrophages are present in most tissues and respond when needed to infections and dying cells. The recognized material is destroyed via phagocytosis in the macrophage, which gives the cells their name (“big eater” in Greek). Macrophages take various forms when present in different locations and can perform additional functions besides phagocytosis.
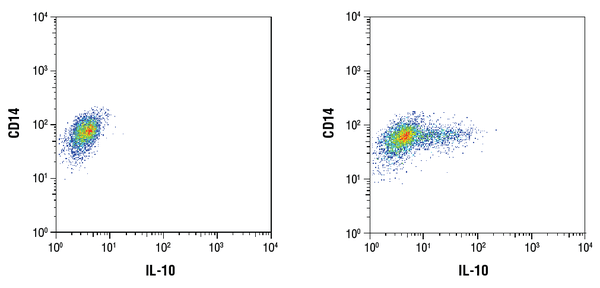
Upon tissue injury or pathogen infection, monocytes in the blood will be recruited to the affected tissue and differentiate to make macrophages. Depending on the tissue localization, different types of macrophages exist, such as Kupffer cells in the liver, alveolar macrophages in the lung, microglia in the brain, etc. These different types of macrophages all come from monocytes but specialize their function to the resident tissue. Most of the general phagocytosis function is carried out by resident tissue macrophages. Besides phagocytosing dead cells and foreign material, macrophages can also signal to other immune cells via cytokines. To a certain extent, macrophages perform the critical function of antigen presentation, accordingly working together with T cells to support adaptive immunity. Additionally, macrophages can secrete cytokines such as IL-12 and play a role in local immune responses, while others secrete high amounts of IL-10, which mediates their role in tissue repair. Thus the “big eaters” play a variety of roles in the immune system in addition to the main job of phagocytosis.
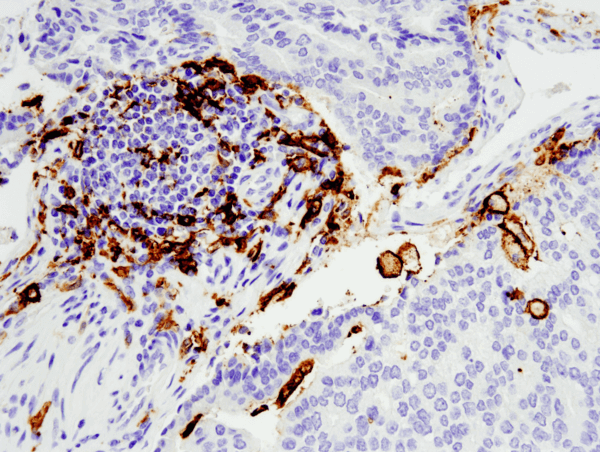
Similar to macrophages, dendritic cells have the capacity to engulf cellular and foreign material but instead of digesting it for removal, dendritic cells process the material and present it as antigens to T cells. As such, dendritic cells serve as antigen-presenting cells, or accessory cells, and thus relay information about pathogens between the innate and adaptive immune systems. Dendritic cells get their name from their distinct cellular projections that reach out and grab harmful material to be digested.
Arising from hematopoietic bone marrow progenitors, initially immature dendritic cells go through a “learning” phase where they sample their local area for foreign material and initiate a recognition response via pattern recognition receptors (one common set of these receptors are the toll-like receptors). These immature dendritic cells can reside in different locations with access to the external environment, such as the lining of the nose, stomach, lungs, and even in the skin. Upon recognition of the antigen, dendritic cells will then migrate to the lymph node where they will train the T cells to recognize the antigen and respond accordingly. Dendritic cells are the only antigen presenting cells to activate both memory and naïve T cells. Toll-like receptors are a well-studied class of receptors present on macrophages and dendritic cells, which mediate the recognition and response to foreign pathogens. Besides interacting directly with T cells, dendritic cells can also signal via cytokines such as IL-12, which acts to ready the immune system for the subsequent attack on the harmful material. Other types of dendritic cells may secrete cytokines that signal to recruit more macrophages to clean up an overload of waste material. As the main cell mediators for antigen presentation, dendritic cells thus play an indispensable role in crosstalk between the innate and adaptive immune systems.
Other cells of the innate immune system include mast cells, which function in wound healing and help provide a protection against pathogens. When triggered by a pathogen, mast cells can secrete cytokines as well as granules containing histamine and heparin, which mediate further immune response such as macrophage recruitment and blood vessel dilation. Neutrophils, eosinophilis, and basophils are collectively referred to as granulocytes due to the characteristic granules in their cytoplasm. In neutrophils, the granules act to release toxic compounds that target pathogens such as fungi and bacteria.
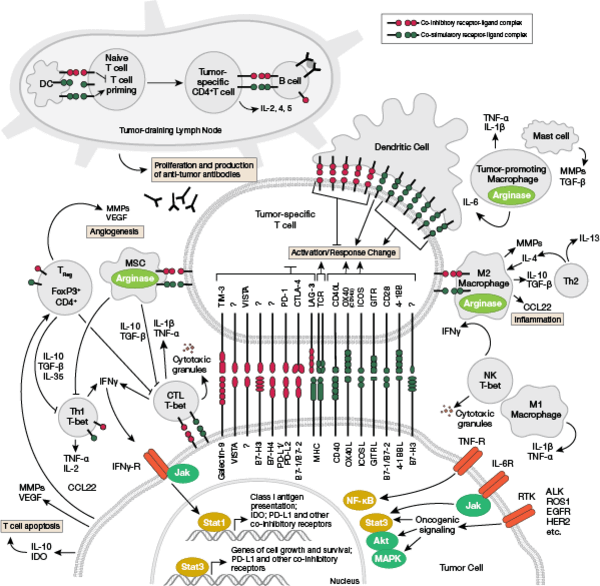
Harnessing immune cells to fight cancer has become a very hot research topic due to success in the clinic. A recent focus of current immunotherapy involves targeting the signaling checkpoint proteins, such as PD-1, that normally prevent T cell activation and response. Adoptive cell therapy is another exciting approach that involves immunoediting T cells harvested from the patient. The T cells are engineered to express a specific chimeric antigen receptor that is specific for the patient’s cancer cells. The T cells are then reinfused into the patient and are now programmed to seek out the cancer cells and kill them, according to the normal T cell function. These and other approaches to manipulate the immune system to attack the harmful cancer cells of the patient represent a ground-breaking class of therapies to treat cancer.