| Antibodies Included | Quantity | Application | Dilution |
|---|---|---|---|
| Oct-4A (C30A3C1) Rabbit mAb (ChIP Formulated) #5677 | 10 immunoprecipitations | ChIP | 1:50 |
| Sox2 (D6D9) XP® Rabbit mAb (ChIP Formulated) #5024 | 10 immunoprecipitations | ChIP | 1:50 |
| Nanog (D73G4) XP® Rabbit mAb (ChIP Formulated) #5232 | 10 immunoprecipitations | ChIP | 1:50 |
| Primers Included | Quantity | Application | Dilution |
|---|---|---|---|
| SimpleChIP® Human Oct-4 Promoter Primers #4641 | 250 PCR reactions | ChIP | 1:10 |
| SimpleChIP® Human α Satellite Repeat Primers #4486 | 250 PCR reactions | ChIP | 1:10 |
ChIP
Product Information
Product Usage Information
Directions for Use:A. Chromatin Immunoprecipitation:ChIP formulated antibodies have been tested and optimized using the SimpleChIP® Enzymatic Chromatin IP Kits (#9002 and #9003). Antibodies should be used at a dilution of 1:50 in a 500 μl ChIP reaction containing 10 to 15 μg of chromatin (4x106 cells). For the SimpleChIP® Enzymatic Chromatin IP protocol, please see the web page for this product at www.cellsignal.com.B. Quantification of DNA by qPCR:1. Label the appropriate number of PCR tubes or PCR plates compatible with the model of real-time PCR machine to be used. PCR reactions should be performed in duplicate and should include a tube with no DNA to control for contamination, and a serial dilution of a 2% total input chromatin DNA (undiluted, 1:5, 1:25, 1:125), which is used to create a standard curve and determine amplification efficiency.2. Add 2 μl of the appropriate ChIP DNA sample to each tube or well of the PCR plate.3. Prepare a master PCR reaction mix as described below. Add enough reagents for two extra reactions to account for loss of volume. Add 18 μl of the master PCR reaction mix to each PCR reaction tube or well of the PCR plate.Reagent Volume for 1 PCR Reaction (20 μl)
Nuclease-free H2O 6 μl
5 μM SimpleChIP® Primers 2 μl
2X SYBR-Green Reaction Mix 10 μl
4. Start the following PCR reaction program:
a. Initial Denaturation 95°C for 3 min
b. Denaturation 95°C for 15 sec
c. Anneal and Extension *Primer-specific temp. for 60 sec
d. Repeat steps b and c for a total of 40 cycles.*65 °C Anneal/Extension for SimpleChIP® Human Oct-4 Promoter Primers*60 °C Anneal/Extension for SimpleChIP® Human α Satellite Repeat Primers5. Analyze quantitative PCR results using software provided with the real-time PCR machine.
Storage
Primers are supplied in nuclease-free water at a concentration of 5 μM and should be stored at -20°C.
Specificity / Sensitivity
Species Reactivity:
Human
Source / Purification
Oct-4 (C30A3C1) XP® Rabbit mAb (ChIP Formulated) is produced by immunizing animals with recombinant protein specific to the amino terminus of human Oct-4A protein. Sox2 (D6D9) XP® Rabbit mAb (ChIP Formulated) is produced by immunizing animals with a synthetic peptide corresponding to residues surrounding Gly179 of human Sox2 protein. Nanog (D73G4) XP® Rabbit mAb (ChIP Formulated) is produced by immunizing animals with a synthetic peptide corresponding to the amino terminus of human Nanog protein.
Product Description
Background
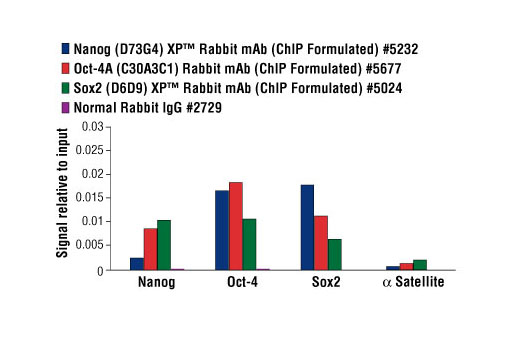
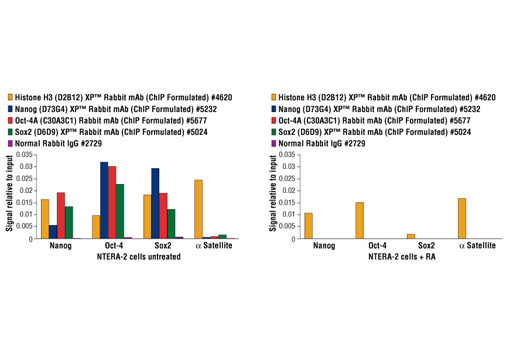
Embryonic stem cells are pluripotent cells derived from the inner cell mass (ICM) of the mammalian blastocyst. Pluripotent cells are capable of indefinite self-renewing expansion in culture and can differentiate into cell types of all three germ layers: endoderm, ectoderm and mesoderm. This pluripotent state is a property shared by embryonic stem (ES) cells, embryonic carcinoma and induced pluripotent stem (iPS) cells. Oct-4, Sox2 and Nanog are key transcriptional regulators that are highly expressed in pluripotent cells (1). Together they form a master transcriptional regulatory network that maintains cells in a pluripotent state (2,3). Over-expression of Oct-4 and Sox2 along with Klf4 and c-Myc can induce pluripotency in both mouse and human somatic cells, highlighting their roles as key regulators of the transcriptional network necessary for self-renewal and pluripotency (4,5). It has also been demonstrated that overexpression of Oct-4, Sox2, Nanog and Lin28 can induce pluripotency in human somatic cells (6). Chromatin immunoprecipitation (ChIP) is a powerful technique that can be used to identify Oct-4, Sox2 and Nanog target genes in a given population of pluripotent cells (2,3,7-9). In addition, ChIP can be used to characterize changes in target gene occupancy that occur during induction of iPS cells from somatic cells, or differentiation of pluripotent cells into different cell lineages.
- Looijenga, L.H. et al. (2003) Cancer Res 63, 2244-50.
- Boyer, L.A. et al. (2005) Cell 122, 947-56.
- Loh, Y.H. et al. (2006) Nat Genet 38, 431-40.
- Takahashi, K. and Yamanaka, S. (2006) Cell 126, 663-76.
- Okita, K. et al. (2007) Nature 448, 313-7.
- Yu, J. et al. (2007) Science 318, 1917-20.
- Okumura-Nakanishi, S. et al. (2005) J Biol Chem 280, 5307-17.
- Catena, R. et al. (2004) J Biol Chem 279, 41846-57.
- Rodda, D.J. et al. (2005) J Biol Chem 280, 24731-7.
Species Reactivity
Species reactivity is determined by testing in at least one approved application (e.g., western blot).
Applications Key
ChIP: Chromatin IP
Cross-Reactivity Key
H: human M: mouse R: rat Hm: hamster Mk: monkey Vir: virus Mi: mink C: chicken Dm: D. melanogaster X: Xenopus Z: zebrafish B: bovine Dg: dog Pg: pig Sc: S. cerevisiae Ce: C. elegans Hr: horse GP: Guinea Pig Rab: rabbit All: all species expected
Trademarks and Patents
Limited Uses
Except as otherwise expressly agreed in a writing signed by a legally authorized representative of CST, the following terms apply to Products provided by CST, its affiliates or its distributors. Any Customer's terms and conditions that are in addition to, or different from, those contained herein, unless separately accepted in writing by a legally authorized representative of CST, are rejected and are of no force or effect.
Products are labeled with For Research Use Only or a similar labeling statement and have not been approved, cleared, or licensed by the FDA or other regulatory foreign or domestic entity, for any purpose. Customer shall not use any Product for any diagnostic or therapeutic purpose, or otherwise in any manner that conflicts with its labeling statement. Products sold or licensed by CST are provided for Customer as the end-user and solely for research and development uses. Any use of Product for diagnostic, prophylactic or therapeutic purposes, or any purchase of Product for resale (alone or as a component) or other commercial purpose, requires a separate license from CST. Customer shall (a) not sell, license, loan, donate or otherwise transfer or make available any Product to any third party, whether alone or in combination with other materials, or use the Products to manufacture any commercial products, (b) not copy, modify, reverse engineer, decompile, disassemble or otherwise attempt to discover the underlying structure or technology of the Products, or use the Products for the purpose of developing any products or services that would compete with CST products or services, (c) not alter or remove from the Products any trademarks, trade names, logos, patent or copyright notices or markings, (d) use the Products solely in accordance with CST Product Terms of Sale and any applicable documentation, and (e) comply with any license, terms of service or similar agreement with respect to any third party products or services used by Customer in connection with the Products.