16
#P05230
2246
Background
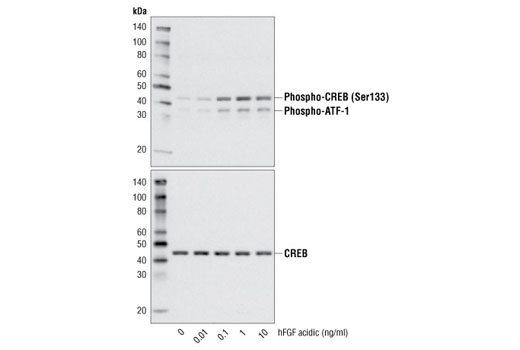
FGF acidic is a potent growth factor for fibroblasts and endothelial cells (1). FGF acidic is involved in wound repair, angiogenesis, and development (1). FGF acidic is secreted from cells via an endoplasmic reticulum/Golgi independent mechanism (1,2). The ability of FGF acidic to bind to heparin sulfate is required for its ability to interact with FGF receptors and induce signaling (1-4). There are four distinct FGF receptors and each has multiple splice variants (1,3). FGF acidic binds with high affinity to many, but not all, FGFRs (1). Signaling cascades activated through FGF basic binding to FGFR include the ras-raf-MAPK, PLCγ/PKC, and PI3K/Akt pathways (1).
Endotoxin
Less than 0.01 ng endotoxin/1 μg hFGF acidic.
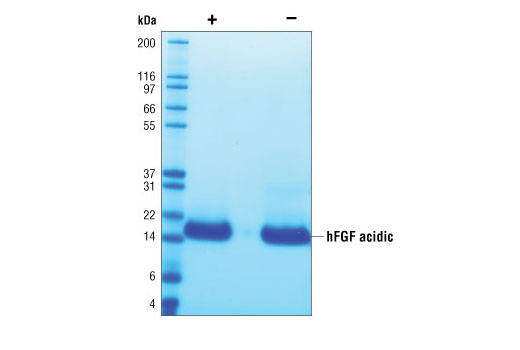
Purity
>98% as determined by SDS-PAGE of 6 μg reduced (+) and non-reduced (-) recombinant hFGF acidic. All lots are greater than 98% pure.
Source / Purification
Recombinant human FGF acidic (hFGF acidic) Phe16-Asp155 (Accession #NP_000791) was produced in E.coli at Cell Signaling Technology.
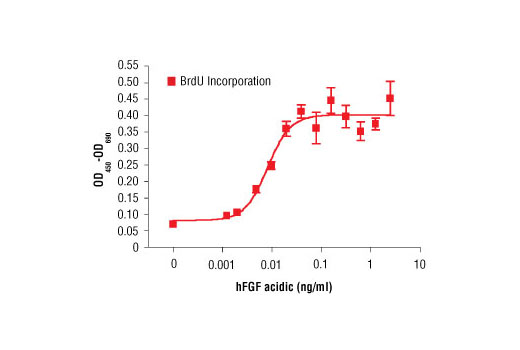
Bioactivity
The bioactivity of recombinant hFGF acidic was determined in a NIH/3T3 cell proliferation assay. The ED50 of each lot is between 7-20 pg/ml.
Background
FGF acidic is a potent growth factor for fibroblasts and endothelial cells (1). FGF acidic is involved in wound repair, angiogenesis, and development (1). FGF acidic is secreted from cells via an endoplasmic reticulum/Golgi independent mechanism (1,2). The ability of FGF acidic to bind to heparin sulfate is required for its ability to interact with FGF receptors and induce signaling (1-4). There are four distinct FGF receptors and each has multiple splice variants (1,3). FGF acidic binds with high affinity to many, but not all, FGFRs (1). Signaling cascades activated through FGF basic binding to FGFR include the ras-raf-MAPK, PLCγ/PKC, and PI3K/Akt pathways (1).
Background References
Cross-Reactivity Key
H: human M: mouse R: rat Hm: hamster Mk: monkey Vir: virus Mi: mink C: chicken Dm: D. melanogaster X: Xenopus Z: zebrafish B: bovine Dg: dog Pg: pig Sc: S. cerevisiae Ce: C. elegans Hr: horse GP: Guinea Pig Rab: rabbit All: all species expected
Trademarks and Patents
Limited Uses
Except as otherwise expressly agreed in a writing signed by a legally authorized representative of CST, the following terms apply to Products provided by CST, its affiliates or its distributors. Any Customer's terms and conditions that are in addition to, or different from, those contained herein, unless separately accepted in writing by a legally authorized representative of CST, are rejected and are of no force or effect.
Products are labeled with For Research Use Only or a similar labeling statement and have not been approved, cleared, or licensed by the FDA or other regulatory foreign or domestic entity, for any purpose. Customer shall not use any Product for any diagnostic or therapeutic purpose, or otherwise in any manner that conflicts with its labeling statement. Products sold or licensed by CST are provided for Customer as the end-user and solely for research and development uses. Any use of Product for diagnostic, prophylactic or therapeutic purposes, or any purchase of Product for resale (alone or as a component) or other commercial purpose, requires a separate license from CST. Customer shall (a) not sell, license, loan, donate or otherwise transfer or make available any Product to any third party, whether alone or in combination with other materials, or use the Products to manufacture any commercial products, (b) not copy, modify, reverse engineer, decompile, disassemble or otherwise attempt to discover the underlying structure or technology of the Products, or use the Products for the purpose of developing any products or services that would compete with CST products or services, (c) not alter or remove from the Products any trademarks, trade names, logos, patent or copyright notices or markings, (d) use the Products solely in accordance with CST Product Terms of Sale and any applicable documentation, and (e) comply with any license, terms of service or similar agreement with respect to any third party products or services used by Customer in connection with the Products.