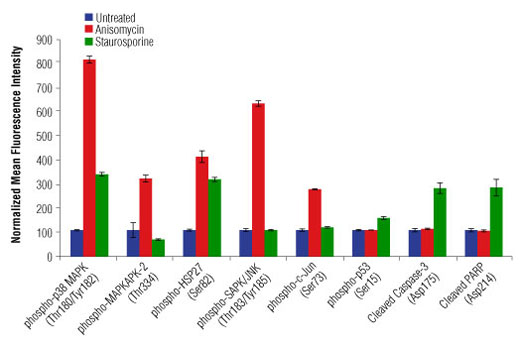
| Kit Includes* | Quantity | Applications | Dilution | Isotype |
|---|---|---|---|---|
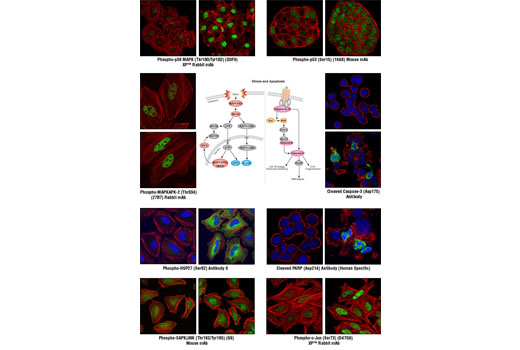
| Phospho-p38 MAPK (Thr180/Tyr182) (D3F9) XP™ Rabbit mAb | 140 µl | HCA, ICW, IF-IC | 1:10 | Rabbit IgG |
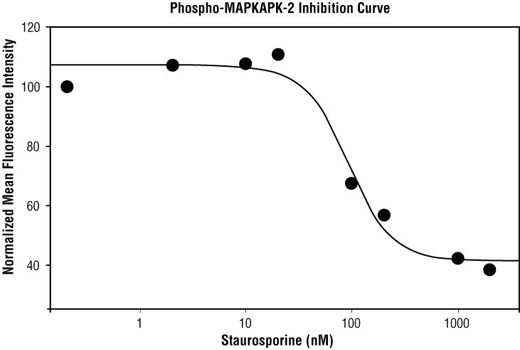
| Phospho-MAPKAPK-2 (Thr334) (27B7) Rabbit mAb | 140 µl | HCA, ICW, IF-IC | 1:10 | Rabbit IgG |
| Phospho-HSP27 (Ser82) Antibody II | 140 µl | HCA, ICW, IF-IC | 1:10 | Rabbit IgG |
| Phospho-SAPK/JNK (Thr183/Tyr185) (G9) Mouse mAb | 140 µl | HCA, ICW, IF-IC | 1:10 | Mouse IgG1 |
| Phospho-c-Jun (Ser73) (D47G9) XP™ Rabbit mAb | 140 µl | HCA, ICW, IF-IC | 1:10 | Rabbit IgG |
| Phospho-p53 (Ser15) (16G8) Mouse mAb | 140 µl | HCA, ICW, IF-IC | 1:10 | Mouse IgG1 |
| Cleaved Caspase-3 (Asp175) Antibody | 140 µl | HCA, ICW, IF-IC | 1:10 | Rabbit IgG |
| Cleaved PARP (Asp214) Antibody (Human Specific) | 140 µl | HCA, ICW, IF-IC | 1:10 | Rabbit IgG |
*Component formulation specific to kit.
Applications Key: HCA=High Content Analysis, ICW=In-Cell Western, IF-IC=Immunofluorescence (Immunocytochemistry)
Description
CST’s PathScan® Multi-Target HCA Stress and Apoptosis Kit contains eight primary antibodies that target cellular stress and apoptotic signaling pathways. This kit is designed to elucidate the signaling occurring through key pathway nodes using automated imaging or laser scanning platforms or manual immunofluorescent microscopy. The kit provides the investigator with a quick and easy means to choose the endpoints that will be the most robust and useful for subsequent studies, whether large high content/high throughput screening projects or single small-scale experiments. The antibodies are supplied at 10X of their optimal dilution for immunofluorescent applications. This allows the antibodies to be easily diluted to their 1X working concentrations and dispensed into multi-well plates or slides. 140 μl of each antibody is supplied, which is sufficient for 24 wells on 96-well plates (50 μl 1X per well) or one row on two 96-well plates.
Storage
Background
Cellular stress and apoptosis involve a complex network of signaling pathways that maintain cellular homeostasis when confronted with a variety of potentially damaging effectors, including UV and gamma radiation, chemotherapeutic agents, osmotic shock, inflammatory cytokines, and other environmental stresses. The manner in which cells respond to stress has become an important metric in the study of disease due to the potential deregulation of these pathways in disease states. For example, cancer cells can affect these pathways to promote cell growth and metastasis (1). Some of the key members involved in stress-activated signaling belong to the mitogen-activated protein kinase (MAPK) pathway. The stress-activated protein kinase/Jun-amino-terminal kinase (SAPK/JNK) is one such member that is potently and preferentially activated by a variety of environmental stresses (2-7). SAPK/JNK, when active as a dimer, can translocate to the nucleus where it regulates transcription through its effects on transcription factors such as c-Jun (4,6). Activation of c-Jun by phosphorylation at Ser63 and Ser73 through SAPK/JNK affects a diverse array of biological functions including cell proliferation, differentiation, and apoptosis (8). Similar to the SAPK/JNK pathway, p38 MAPK is activated by a variety of cellular stresses (9-13). When phosphorylated at Thr180 and Tyr182, p38 MAPK has been shown to activate MAP kinase-activated protein kinase 2 (MAPKAPK-2) and the transcription factors ATF-2, Max, and MEF2 (11-16). Phosphorylation at Thr222, Ser272, and Thr334 appears to be essential for the activity of MAPKAPK-2 (17), which can result in the phosphorylation of heat shock protein (HSP) 27 at Ser15, Ser78, and Ser82 (9,18). HSP27 is one of the small HSPs that are constitutively expressed at different levels in various cell types and tissues. In response to stress, the expression level of HSP27 increases several-fold to confer cellular resistance to the adverse environmental change (18). The SAPK/JNK and p38 MAPK pathways also contribute to cell cycle checkpoint control through the activation of the p53 tumor suppressor protein, which plays a major role in cellular response to DNA damage and other genomic aberrations (19). Activation of p53 can lead to either cell cycle arrest and DNA repair or apoptosis (20). Stress-activated pathways also control the transcription of apoptotic proteins and mediators, thereby playing an important role in apoptosis and cell survival. Apoptosis is a regulated cellular suicide mechanism characterized by nuclear condensation, cell shrinkage, membrane blebbing, and DNA fragmentation (21). Cell survival requires the active suppression of apoptosis, which is accomplished by inhibiting the expression of pro-apoptotic factors as well as promoting the expression of anti-apoptotic factors. Caspases, a family of cysteine proteases, are the central regulators of apoptosis. Initiator caspases (including caspase-2, -8, -9, -10, -11, and -12) are closely coupled to pro-apoptotic signals. Once activated, these caspases cleave and activate downstream effector caspases (including caspase-3, -6, and -7), which in turn execute apoptosis by cleaving cellular proteins following specific asparagine residues (1). Caspase-3 is a critical executioner of apoptosis, as it is either partially or totally responsible for the proteolytic cleavage of many key proteins such as the nuclear enzyme poly (ADP-ribose) polymerase (PARP) (22). PARP appears to be involved in DNA repair in response to environmental stress (23). PARP helps cells to maintain their viability; cleavage of PARP facilitates cellular disassembly and serves as a marker of cells undergoing apoptosis (24).
- Herr, I. and Debatin, K.M. (2001) Blood 98, 2603-14.
- Davis, R.J. (1999) Biochem Soc Symp 64, 1-12.
- Ichijo, H. (1999) Oncogene 18, 6087-93.
- Kyriakis, J.M. and Avruch, J. (2001) Physiol Rev 81, 807-69.
- Kyriakis, J.M. (1999) J Biol Chem 274, 5259-62.
- Leppä, S. and Bohmann, D. (1999) Oncogene 18, 6158-62.
- Whitmarsh, A.J. and Davis, R.J. (1998) Trends Biochem Sci 23, 481-5.
- Davis, R.J. (2000) Cell 103, 239-52.
- Rouse, J. et al. (1994) Cell 78, 1027-37.
- Han, J. et al. (1994) Science 265, 808-11.
- Lee, J.C. et al. Nature 372, 739-46.
- Freshney, N.W. et al. (1994) Cell 78, 1039-49.
- Raingeaud, J. et al. (1995) J Biol Chem 270, 7420-6.
- Zervos, A.S. et al. (1995) Proc Natl Acad Sci U S A 92, 10531-4.
- Zhao, M. et al. (1999) Mol Cell Biol 19, 21-30.
- Yang, S.H. et al. (1999) Mol Cell Biol 19, 4028-38.
- Ben-Levy, R. et al. (1995) EMBO J 14, 5920-30.
- Landry, J. et al. (1992) J Biol Chem 267, 794-803.
- Reinhardt, H.C. and Yaffe, M.B. (2009) Curr Opin Cell Biol 21, 245-55.
- Levine, A.J. (1997) Cell 88, 323-31.
- Elmore, S. (2007) Toxicol Pathol 35, 495-516.
- Fernandes-Alnemri, T. et al. (1994) J Biol Chem 269, 30761-4.
- Satoh, M.S. and Lindahl, T. (1992) Nature 356, 356-8.
- Oliver, F.J. et al. (1998) J Biol Chem 273, 33533-9.
Background References
Trademarks and Patents
Limited Uses
Except as otherwise expressly agreed in a writing signed by a legally authorized representative of CST, the following terms apply to Products provided by CST, its affiliates or its distributors. Any Customer's terms and conditions that are in addition to, or different from, those contained herein, unless separately accepted in writing by a legally authorized representative of CST, are rejected and are of no force or effect.
Products are labeled with For Research Use Only or a similar labeling statement and have not been approved, cleared, or licensed by the FDA or other regulatory foreign or domestic entity, for any purpose. Customer shall not use any Product for any diagnostic or therapeutic purpose, or otherwise in any manner that conflicts with its labeling statement. Products sold or licensed by CST are provided for Customer as the end-user and solely for research and development uses. Any use of Product for diagnostic, prophylactic or therapeutic purposes, or any purchase of Product for resale (alone or as a component) or other commercial purpose, requires a separate license from CST. Customer shall (a) not sell, license, loan, donate or otherwise transfer or make available any Product to any third party, whether alone or in combination with other materials, or use the Products to manufacture any commercial products, (b) not copy, modify, reverse engineer, decompile, disassemble or otherwise attempt to discover the underlying structure or technology of the Products, or use the Products for the purpose of developing any products or services that would compete with CST products or services, (c) not alter or remove from the Products any trademarks, trade names, logos, patent or copyright notices or markings, (d) use the Products solely in accordance with CST Product Terms of Sale and any applicable documentation, and (e) comply with any license, terms of service or similar agreement with respect to any third party products or services used by Customer in connection with the Products.