SignalStar Multiplex IHC is an advanced technology for labeling multiple cellular proteins simultaneously in FFPE tissue samples using specific primary antibodies and fluorescent detection reagents. It offers high accuracy and reliability in visualizing and analyzing protein expression in tissue samples.
Because CST validates all of our antibodies in each application independently, SignalStar Multiplex IHC antibodies are only recommended when we have validated them specifically for use with SignalStar technology. That means that you can be confident that your SignalStar antibody will work as expected in your assay every time, guaranteed.
We provide expert support to optimize the application of SignalStar Multiplex IHC technology to reduce the time in your research. Our team is available to answer questions and offer technical assistance.
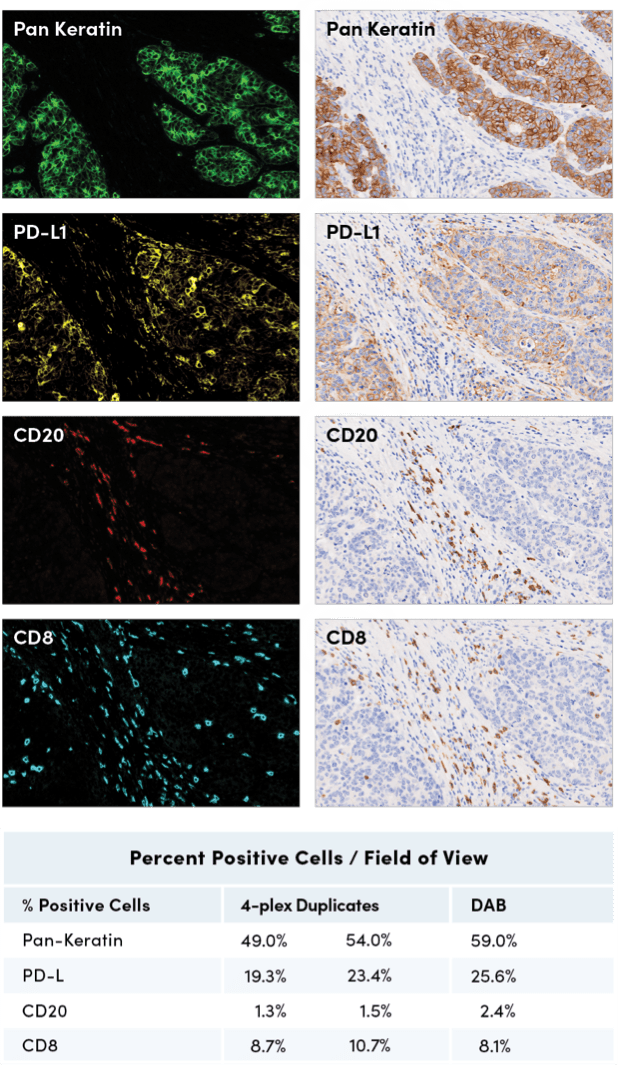
SignalStar multiplex immunohistochemical analysis of paraffin-embedded human gastric adenocarcinoma, using Pan-Keratin (C11) & C0-0003-488 SignalStar Oligo-Antibody Pair #63566 (top left, green), PD-L1 (E1L3N) & C0-0005-594 SignalStar Oligo-Antibody Pair #28249 (upper middle left, yellow), CD20 (E7B7T) & C0-0011-647 SignalStar Oligo-Antibody Pair #36775 (lower middle left, red), and CD8α (D8A8Y) & C0-0004-750 SignalStar Oligo-Antibody Pair #62750 (bottom left, cyan), compared to chromogenic immunohistochemical analysis of serial sections of paraffin-embedded human gastric adenocarcinoma using Pan-Keratin (C11) Mouse mAb #4545 (top right), PD-L1 (E1L3N) XP® Rabbit mAb #13684 (upper middle right), CD20 (E7B7T) XP® Rabbit mAb #48750 (lower middle right), and CD8α (D8A8Y) Rabbit mAb #85336 (bottom right). All fluorophores have been assigned a pseudocolor, as indicated. All protocols were performed on the BOND RX Fully Automated Research Stainer by Leica Biosystems. Percent positive cells were quantified in matched regions of interest from serial sections for the SignalStar assay (n=2) compared to the chromogenic.
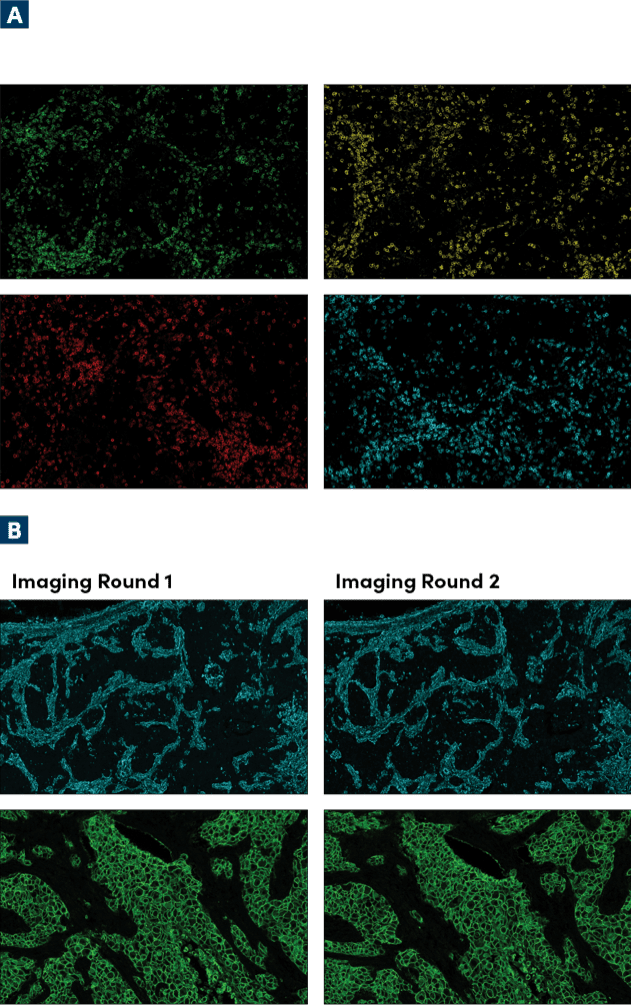
A) SignalStar immunohistochemical analysis of paraffin-embedded human non-small cell lung carcinoma, using CD3ε (D7A6E) & C0-0001- 488 SignalStar Oligo-Antibody Pair #92856 (top left, green), CD3ε (D7A6E) & C0-0001-594 SignalStar Oligo-Antibody Pair #84634 (top right, yellow), CD3ε (D7A6E) & C0-0001-647 SignalStar Oligo-Antibody Pair #33888 (bottom left, red), CD3ε (D7A6E) & C0-0001-750 SignalStar Oligo-Antibody Pair #51754 (bottom right, cyan). All fluorophores have been assigned a pseudocolor, as indicated. Staining was performed on the BOND RX autostainer. B) SignalStar immunohistochemical analysis in serial sections of paraffin-embedded human infiltrating ductal carcinoma of the breast, the first imaging round (left) compared to the second imaging round (right), using Vimentin (D21H3) CO-0012-750 SignalStar Oligo-Antibody Pair #61002 (top panel, cyan) or HER2/ErbB2 (D8F12) & CO-0019-488 SignalStar Oligo-Antibody Pair #31149 (bottom panel, green). All fluorophores have been assigned a pseudocolor, as indicated. Staining was performed on the BOND RX autostainer.
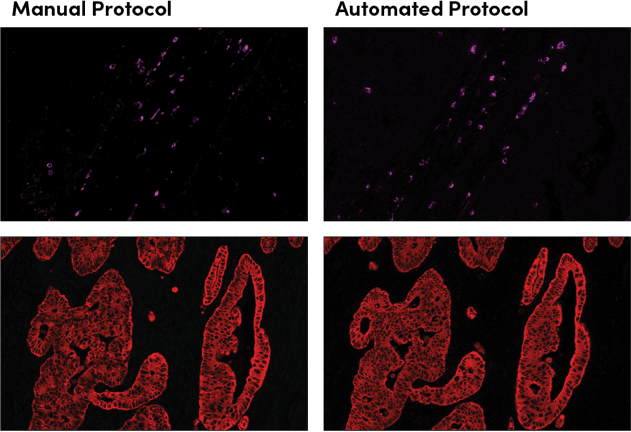
SignalStar immunohistochemical analysis in serial sections of paraffin-embedded human colorectal adenocarcinoma, with staining performed using the manual protocol (left) compared to the Bond RX protocol (right), using Granzyme B (D6E9W) & CO-0009-647 SignalStar Oligo-Antibody Pair #37369 (magenta) or Pan-Keratin (C11) & C0-0003-647 SignalStar Oligo-Antibody Pair #16373 (red). All fluorophores have been assigned a pseudocolor, as indicated.