Stem Cell & Lineage Markers
CST signaling pathway diagrams allow you to click on individual nodes to find research resources or product information. You can also download the pathway diagrams for educational and research purposes.
Moments after conception, a fertilized egg (zygote) initiates a highly regulated program of proliferation and directed differentiation known as embryogenesis. The development events that occur during embryogenesis, which are guided by complex genetic and epigenetic signaling cascades, encompass the first stages of a highly complex process that ultimately results in the generation of a new, fully-formed multi-cellular organism.
Embryonic stem cells (ESCs) are a defined cell population derived from the inner cell mass of the cleavage-stage embryo (blastocyst). The defining characteristics of ESCs are their inherent pluripotency, which allows them to differentiate into any cell lineage of the body, and their potential for indefinite self-renewal. These traits, which are tightly regulated by a complex array of cell signaling networks, combine to make ESCs a powerful tool for research in developmental biology, with significant potential in personalized regenerative medicine.
In humans, the primary signaling pathways responsible for maintaining pluripotency and self renewal in ESCs are the BMP/TGF-β signaling pathway, which signals through SMAD proteins, and the FGF signaling pathway, which activates the MAPK and Akt pathways. The Wnt signaling pathway also promotes pluripotency, although this may occur through a non-canonical mechanism involving a balance between the transcriptional activator TCF1 and the repressor TCF3. Signaling through these pathways results in the expression and activation of three key transcription factors: OCT-4, SOX2, and NANOG. These transcription factors promote the expression of ESC-specific genes, regulate their own expression, and serve as useful markers of pluripotency. Other markers used to identify hESCs include the cell surface glycolipid SSEA3/4 and glycoproteins TRA-1-60 and TRA-1-81.
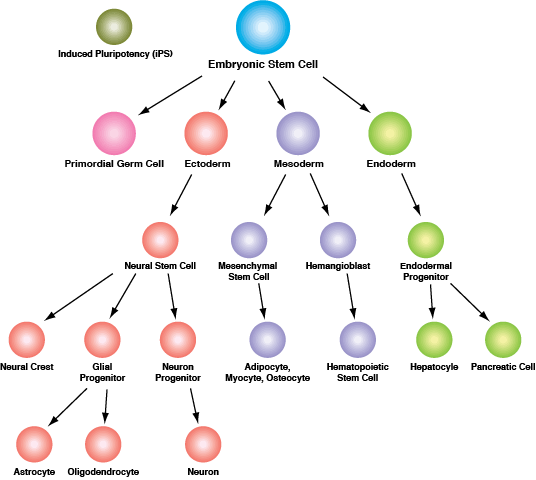
Induced pluripotent stem cells (iPSCs) are pluripotent, ESC-like cells that can be derived from differentiated cells by forced expression of a defined set of “reprogramming” factors, the best known of which are OCT-4, SOX2, KLF4, and c-MYC. Following successful reprogramming, iPSCs exhibit a gene expression signature similar to that of ESCs and exhibit both pluripotency and the capacity of self-renewal. They have consequently garnered much attention from the research community, as their use in research avoids much of the inherent ethical and technical issues surrounding the use of ESCs derived from human blastocysts. Like ESCs, iPSCs are the subject of intense investigation, due to their enormous potential for use in regenerative and personalized medicine, drug screening, and to further our understanding of the cell signaling networks that regulate embryonic development.
Both ESCs and iPSCs can be induced to develop into distinct cell types, representing each of the three primary germ layers that are established during gastrulation: ectoderm, mesoderm, and endoderm. The ectoderm is the precursor of neuronal stem cells, which divide to yield the cells comprising the brain, spinal cord and peripheral nerves. Other cells of ectodermal origin include the epidermis, and distal regions of the digestive tract. The mesoderm differentiates into mesenchymal stem cells, the precursors to fat, muscle and bone, and hematopoietic stem cells, which yield all the cell lineages of the blood and immune systems. The endoderm differentiates into endodermal progenitor cells (the precursor of both hepatic and pancreatic cells) and is also the precursor of the cells that line the digestive, respiratory and urinary tracts.
Development along each lineage is regulated by several signaling pathways that control cell division, growth and differentiation, including BMP/TGF-β, Notch, Wnt/β-catenin, Hedgehog, and Hippo pathways. Each of these pathways is regulated by a complex array of genetic, epigenetic (e.g., histone modification) and exogenous signaling factors that serve to guide cell fate and behavior during development and differentiation.
References:
- Wilson CW, Chuang PT (2010) Mechanism and evolution of cytosolic Hedgehog signal transduction. Development 137(13), 2079–94.
- Young RA (2011) Control of the embryonic stem cell state. Cell 144(6), 940–54.
- Ng HH, Surani MA (2011) The transcriptional and signalling networks of pluripotency. Nat. Cell Biol. 13(5), 490–6.
- Miki T, Yasuda SY, Kahn M (2011) Wnt/β-catenin signaling in embryonic stem cell self-renewal and somatic cell reprogramming. Stem Cell Rev 7(4), 836–46.
- Orkin SH, Hochedlinger K (2011) Chromatin connections to pluripotency and cellular reprogramming. Cell 145(6), 835–50.
- Zhao B, Tumaneng K, Guan KL (2011) The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nat. Cell Biol. 13(8), 877–83.
- Andersson ER, Sandberg R, Lendahl U (2011) Notch signaling: simplicity in design, versatility in function. Development 138(17), 3593–612.
- Evans M (2011) Discovering pluripotency: 30 years of mouse embryonic stem cells. Nat. Rev. Mol. Cell Biol. 12(10), 680–6.
- Watanabe K, Dai X (2011) A WNTer revisit: new faces of β-catenin and TCFs in pluripotency. Sci Signal 4(193), pe41.
- Robinton DA, Daley GQ (2012) The promise of induced pluripotent stem cells in research and therapy. Nature 481(7381), 295–305.