Autophagy Research Resources
CST signaling pathway diagrams allow you to click on individual nodes to find research resources or product information. You can also download the pathway diagrams for educational and research purposes.
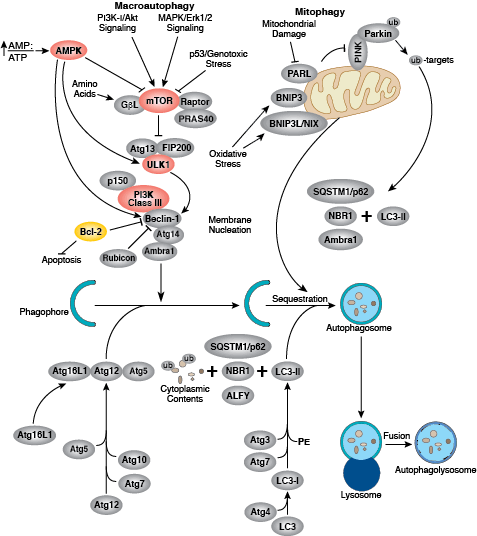
Autophagy is a dynamic cellular recycling system that degrades cytoplasmic contents, abnormal protein aggregates, and excess or damaged organelles so that the building blocks, such as amino acids, can be used to create new cellular components. Autophagy occurs when the protein, organelle, or cytoplasmic content to be degraded is surrounded by a small portion of membrane, creating an autophagosome. The autophagosome is then fused to the lysosome, creating an autolysosome and resulting in degradation of cellular components via lysosomal enzymes. Autophagy is generally activated by conditions of nutrient deprivation but has also been associated with physiological as well as pathological processes such as development, differentiation, neurodegenerative diseases, stress, infection, obesity, and cancer.
The kinase mTOR is a critical regulator of autophagy induction, with activated mTOR (Akt and MAPK signaling) suppressing autophagy, and negative regulation of mTOR (AMPK and p53 signaling) promoting it. The molecular machinery of autophagy was largely discovered in yeast and is directed by a number of autophagy-related (Atg) genes. The serine/threonine kinase ULK plays a similar role as the yeast Atg1, acting downstream of the mTOR complex. ULK forms a large complex with Atg13 and the scaffold protein FIP200. The class III type phosphoinositide 3-kinase (PI3K class III or hVps34) is a major regulator of autophagy. Multiple proteins associate with PI3K class III, including p105/Vsp15, Beclin-1, UVRAG, Atg14, and Rubicon. Rubicon inhibits PI3K class III lipid kinase activity and opposes the action of Atg14L, an enhancer of PI3K class III activity. The Atg genes control the autophagosome formation through Atg12-Atg5 and LC3-II (Atg8-II) complexes. Atg12 is conjugated to Atg5 in a ubiquitin-like reaction that requires Atg7 and Atg10 (E1 and E2-like enzymes, respectively). The Atg12–Atg5 conjugate then interacts noncovalently with Atg16 to form a large complex. The second complex, LC3/Atg8, is cleaved at its C-terminus by Atg4 protease to generate the cytosolic LC3-I. LC3-I is conjugated to phosphatidylethanolamine (PE) in a ubiquitin-like reaction that requires Atg7 and Atg3 (E1 and E2-like enzymes, respectively). The lipidated form of LC3, known as LC3-II, is attached to the autophagosome membrane. Sequestosome 1 (SQSTM1, p62) is a ubiquitin binding protein that binds LC3/Atg8, thereby promoting autophagy by bringing SQSTM1-containing protein aggregates to the autophagosome for degradation.
References:
- Mizushima N, Yoshimori T, Ohsumi Y (2011) The role of Atg proteins in autophagosome formation. Annu. Rev. Cell Dev. Biol. 27, 107–32.
- Rubinsztein DC, Maria G, Kroemer G (2011) Autophagy and aging. Cell 146(5), 682–95.
- Kimmelman AC (2011) The dynamic nature of autophagy in cancer. Genes Dev. 25(19), 1999–2010.
- Sridhar S, Botbol Y, Macian F, Cuervo AM (2012) Autophagy and disease: always two sides to a problem. J. Pathol. 226(2), 255–73.
- Alers S, Luffler AS, Wesselborg S, Stork B (2012) Role of AMPK-mTOR-Ulk1/2 in the regulation of autophagy: cross talk, shortcuts, and feedbacks. Mol. Cell. Biol. 32(1), 2–11.
- Moscat J, Diaz-Meco MT (2011) Feedback on fat: p62-mTORC1-autophagy connections. Cell 147(4), 724–7.
- Mizushima N, Komatsu M (2011) Autophagy: renovation of cells and tissues. Cell 147(4), 728–41.
- Codogno P, Mehrpour M, Proikas-Cezanne T (2012) Canonical and non-canonical autophagy: variations on a common theme of self-eating? Nat. Rev. Mol. Cell Biol. 13(1), 7–12.
- Lee J, Giordano S, Zhang J (2012) Autophagy, mitochondria and oxidative stress: cross-talk and redox signalling. Biochem. J. 441(2), 523–40.