Cytoskeletal Regulation and Vesicle Trafficking
CST signaling pathway diagrams allow you to click on individual nodes to find research resources or product information. You can also download the pathway diagrams for educational and research purposes.
The cytoskeleton consists of three types of cytosolic fibers: microtubules, microfilaments (actin filaments), and intermediate filaments. Cytoskeletal signaling regulates several important cellular processes such as cell division, adhesion, polarity, migration, and movement through cilia and flagella.
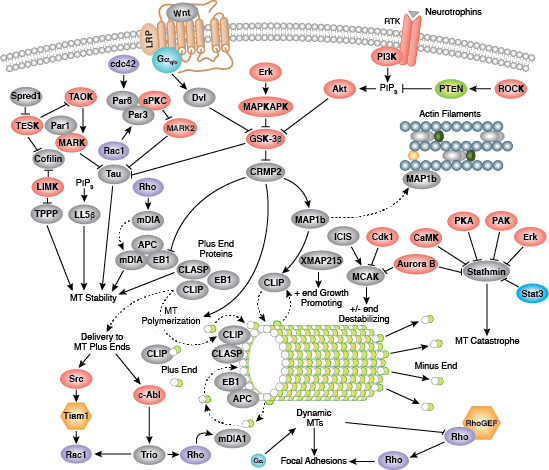
Microtubules are composed of globular tubulin subunits, with α/β−tubulin heterodimers forming the tubulin subunit common to all eukaryotic cells. γ−tubulin is required to nucleate polymerization of tubulin subunits to form microtubule polymers. Many cell movements are mediated by microtubule action, including the beating of cilia and flagella, cytoplasmic transport of membrane vesicles, and nerve-cell axon migration. Microtubules also play a critical role in spindle assembly during mitosis/meiosis and are responsible for chromosome alignment during metaphase. Microtubules form the 9+2 structure of the centriole, a critical component of the centrosome that acts as a microtubule-organizing center (MTOC) and plays a role in cell polarity. Because of their role in mitosis, microtubules have been targets of chemotherapy in cancer. Microtubules continuously undergo a process of dynamic instability, whereby microtubule polymerization on the plus end competes with depolymerization at the minus end. This process is regulated by several signaling molecules including stathmin, diap1/2, tau, and the Rho family of small GTPases.
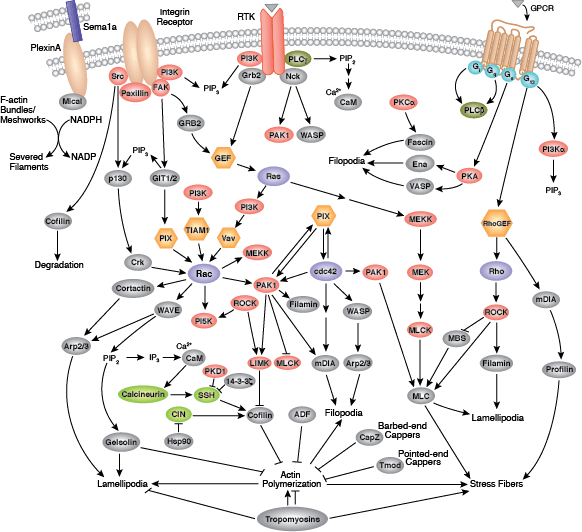
Microfilaments are major structural components of the cytoskeleton and consist of fibrous polymers of actin, F-actin. Microfilaments are important for changes in cell shape, migration, proliferation, and survival. Regulation of the actin cytoskeleton begins with signaling through G protein-coupled receptors (GPCRs), integrins, receptor tyrosine kinases (RTKs), and numerous other specialized receptors, such as the semaphorin 1a receptor PlexinA. These receptors initiate a large number of signaling cascades that include the Rho family of small GTPases (Rho, Rac, and Cdc42) and their activators, guanine nucleotide exchange factors (GEFs), their downstream protein kinase effectors, including Rho-kinase/ROCK and p21 activated kinase (PAK), as well as through direct binding of the GTPases to several actin regulatory proteins, such as cortactin, diap1/2, WAVE, and WASP. These cascades converge on proteins that directly regulate the behavior and organization of the actin cytoskeleton, including actin interacting regulatory proteins such as cofilin, Arp2/3 complex, Ena/VASP, profilin, and gelsolin.
Major types of intermediate filaments are distinguished by their cell-specific expression: cytokeratins (epithelial cells), glial fibrillary acidic protein (GFAP) (glial cells), desmin (skeletal, visceral, and certain vascular smooth muscle cells), vimentin (mesenchyme origin), and neurofilaments (neurons). Members of this group contain a globular amino-terminal head domain, a central α−tubulin rod domain, and a carboxy-terminal tail. Intermediate filaments provide structural support for the cell, act as anchorage points for organelles and molecular motors, and function as stress proteins that provide protection from intrinsic and environmental stresses. Intermediate filaments can be regulated through several mechanisms including phosphorylation, which affects their activity or ability to assemble with other intermediate filament-interacting proteins.
References:
- Goldman RD, Grin B, Mendez MG, Kuczmarski ER (2008) Intermediate filaments: versatile building blocks of cell structure. Curr. Opin. Cell Biol. 20(1), 28–34.
- Toivola DM, Strnad P, Habtezion A, Omary MB (2010) Intermediate filaments take the heat as stress proteins. Trends Cell Biol. 20(2), 79–91.
- Ridley AJ (2011) Life at the leading edge. Cell 145(7), 1012–22.
- Rottner K, Stradal TE (2011) Actin dynamics and turnover in cell motility. Curr. Opin. Cell Biol. 23(5), 569–78.
- Windoffer R, Beil M, Magin TM, Leube RE (2011) Cytoskeleton in motion: the dynamics of keratin intermediate filaments in epithelia. J. Cell Biol. 194(5), 669–78.
- Sandquist JC, Kita AM, Bement WM (2011) And the dead shall rise: actin and myosin return to the spindle. Dev. Cell 21(3), 410–9.
- Field CM, La¡rt P (2011) Bulk cytoplasmic actin and its functions in meiosis and mitosis. Curr. Biol. 21(19), R825–30.
- Aylett CH, Lawe J, Amos LA (2011) New insights into the mechanisms of cytomotive actin and tubulin filaments. Int Rev Cell Mol Biol 292, 1–71.
- Bornens M (2012) The centrosome in cells and organisms. Science 335(6067), 422–6.